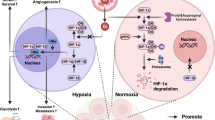
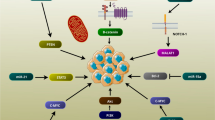
Abstract
As a landmark, scientific investigation in cytokine signaling and interferon-related anti-viral activity, signal transducer and activator of transcription (STAT) family of proteins was first discovered in the 1990s. Today, we know that the STAT family consists of several transcription factors which regulate various molecular and cellular processes, including proliferation, angiogenesis, and differentiation in human carcinoma. STAT family members play an active role in transducing signals from cell membrane to nucleus through intracellular signaling and thus activating gene transcription. Additionally, they are also associated with the development and progression of human cancer by facilitating inflammation, cell survival, and resistance to therapeutic responses. Accumulating evidence suggests that not all STAT proteins are associated with the progression of human malignancy; however, STAT3/5 are constitutively activated in various cancers, including multiple myeloma, lymphoma, breast cancer, prostate hepatocellular carcinoma, and non-small cell lung cancer. The present review highlights how STAT-associated events are implicated in cancer inflammation, angiogenesis and non-coding RNA (ncRNA) modulation to highlight potential intervention into carcinogenesis-related cellular processes.





Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available due to [not applicable for review articles] but are available from the corresponding author on reasonable request.
Abbreviations
- AKT:
-
protein kinase B
- Ang-2:
-
Angiopoietin-2
- APE/Ref-1:
-
Apurinic (apyrimidinic) endonuclease/redox-factor 1
- CPT1B:
-
Carnitine palmitoyltransferase 1B
- CBP:
-
CREB binding protein
- CDKN1A:
-
cyclin dependent kinase inhibitor 1 A
- CRC:
-
Colorectal cancer
- COX-2:
-
cyclooxygenase-2
- DC:
-
dendritic cell
- DBD:
-
DNA binding domain
- DUXAP8:
-
double homeobox A pseudogene 8
- EBV:
-
Epstein-Barr virus
- FAO:
-
fatty acid beta-oxidation
- GRP:
-
glucose-regulated protein
- TDM1:
-
trastuzumab emtansine
- HTLV-1:
-
human T-lymphotropic virus type 1
- HOST2:
-
human ovarian cancer-specific transcript 2
- HIC1:
-
hypermethylation of cancer 1
- HIF-1α:
-
Hypoxia-inducible factor 1-alpha
- IFN:
-
interferon
- IFNAR:
-
interferon alpha receptor
- IL:
-
interleukin
- JAK:
-
Janus kinase
- MLL1:
-
mixed lineage leukaemia protein-1
- MMP:
-
matrix metalloproteinase
- ncRNA:
-
non-coding RNA
- NFκB:
-
nuclear factor kappaB
- NK:
-
natural killer
- Okt4:
-
octamer-binding transcription factor 4
- PD-L1:
-
programmed death-ligand 1
- PI3K:
-
phosphoinositide 3-kinase
- PRL:
-
prolactin
- RN/OS:
-
reactive nitrogen/oxygen species
- SH2:
-
Src-homology 2 (domain)
- SCLC:
-
small cell lung cancer
- SNHG10:
-
small nucleolar RNA host gene 10
- SOC:
-
suppressor of cytokine signaling
- STAT TF:
-
transcription factor
- STAT:
-
signal transducer and activator of transcription
- TAD:
-
transcriptional activation domain
- DBD:
-
DNA-binding domain
- PTP:
-
protein tyrosine phosphatases
- TGF-β:
-
transforming growth factor-beta
- Th:
-
T-helper
- TIMPs:
-
tissue inhibitors of metalloproteinases
- TME:
-
tumor microenvironment
- TNF-α:
-
tumour necrosis factor-alpha
- TRAF:
-
TNF receptor-associated factor
- TYK2:
-
tyrosine kinase 2
- VEGF:
-
vascular endothelial growth factor
References
Kashyap D, Tuli HS, Yerer MB et al (2021) Natural product-based nanoformulations for cancer therapy: Opportunities and challenges. Semin Cancer Biol 69:5–23. https://doi.org/10.1016/j.semcancer.2019.08.014
Valladares BT, Crespo PC, Herranz UA, Caamaño AG (2021) Adjuvant treatment in lung cancer. J Clin Transl Res 7:175–184. https://doi.org/10.18053/jctres.07.202102.012
Luna J, Sotoca A, Fernández P et al (2021) Recent advances in early stage lung cancer. J Clin Transl Res 7:163–174. https://doi.org/10.18053/jctres.07.202102.010
Lu X, Shi H, Que Q, Qiu S (2021) Research progress in immunotherapy of advanced non-small cell lung cancer. Trends Immunother 5:58–64
Furukawa F (2021) Effects of immune checkpoint inhibitors on cancer patients with preexisting autoimmune disease. Trends Immunother 5:5–6
Srivani G, Peela S, Alam A, Purnachandra GN (2021) Gemcitabine for pancreatic cancer. Cancer Plus 3:1–13. https://doi.org/10.18063/CP.V3I3.323
Jinhan Tian Tang SJ (2021) Transferrin Receptor Serves as a Potential Target for Cancer Therapy. https://doi.org/10.18063/CP.V3I2.317. Cancer Plus 3:
Kashyap D, Garg VK, Goel N (2021) Intrinsic and extrinsic pathways of apoptosis: Role in cancer development and prognosis, 1st edn. Elsevier Inc
Turkson J (2004) STAT proteins as novel targets for cancer drug discovery. Expert Opin Ther Targets 8:409–422. https://doi.org/10.1517/14728222.8.5.409
Furqan M, Akinleye A, Mukhi N et al (2013) STAT inhibitors for cancer therapy. J Hematol Oncol 6:90. https://doi.org/10.1186/1756-8722-6-90
Hanada T, Yoshimura A (2002) Regulation of cytokine signaling and inflammation. Cytokine Growth Factor Rev 13:413–421. https://doi.org/10.1016/S1359-6101(02)00026-6
Hoey T, Schindler U (1998) STAT structure and function in signaling. Curr Opin Genet Dev 8:582–587. https://doi.org/10.1016/S0959-437X(98)80015-4
Kurokawa R, Kalafus D, Ogliastro MH et al (1998) Differential use of CREB binding protein-coactivator complexes. Sci (80-) 279:700–703. https://doi.org/10.1126/science.279.5351.700
Lavecchia A, Di Giovanni C, Novellino E (2011) STAT-3 Inhibitors: State of the Art and New Horizons for Cancer Treatment. Curr Med Chem 18:2359–2375. https://doi.org/10.2174/092986711795843218
Shuai K, Liu B (2003) Regulation of JAK-STAT signalling in the immune system. Nat Rev Immunol 3:900–911. https://doi.org/10.1038/nri1226
Alexander WS, Hilton DJ (2004) The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annu Rev Immunol 22:503–529. https://doi.org/10.1146/ANNUREV.IMMUNOL.22.091003.090312
Verhoeven Y, Tilborghs S, Jacobs J et al (2020) The potential and controversy of targeting STAT family members in cancer. Semin Cancer Biol 60:41–56. https://doi.org/10.1016/j.semcancer.2019.10.002
Cohen S (1976) Cell mediated immunity and the inflammatory system. Hum Pathol 7:249–264. https://doi.org/10.1016/S0046-8177(76)80036-6
Ihle JN, Thierfelder W, Teglund S et al (1998) Signaling by the cytokine receptor superfamily. Ann N Y Acad Sci 865:1–9. doi: 10.1111/j.1749-6632.1998.tb11157.x. Erratum in: Ann N Y Acad Sci. 2006 Oct;1078:following 626. Stravapodis, D [corrected to Stravopodis, D]. PMID: 9927991.
Bromberg JF, Horvath CM, Wen Z et al (1996) Transcriptionally active Stat1 is required for the antiproliferative effects of both interferon α and interferon γ. Proc Natl Acad Sci U S A 93:7673–7678. https://doi.org/10.1073/pnas.93.15.7673
Decker T, Stockinger S, Karaghiosoff M et al (2002) IFNs and STATs in innate immunity to microorganisms. J Clin Invest 109:1271–1277. https://doi.org/10.1172/jci15770
Loh CY, Arya A, Naema AF et al (2019) Signal Transducer and Activator of Transcription (STATs) Proteins in Cancer and Inflammation: Functions and Therapeutic Implication. Front Oncol 9. https://doi.org/10.3389/FONC.2019.00048
Loh CY, Arya A, Naema AF et al (2019) Signal transducer and activator of transcription (STATs) proteins in cancer and inflammation: Functions and therapeutic implication. Front Oncol 9:48. https://doi.org/10.3389/fonc.2019.00048
Rauch I, Müller M, Decker T (2013) The regulation of inflammation by interferons and their STATs. JAK-STAT 2:e23820. https://doi.org/10.4161/jkst.23820
Stritesky GL, Kaplan MH (2011) Changing the STATus quo in T helper cells. Transcription 2:179–182. https://doi.org/10.4161/trns.2.4.16614
T T, T K (1997) Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol 15:797–819. https://doi.org/10.1146/ANNUREV.IMMUNOL.15.1.797
HORAK I, Lohler J, MA A, SMITH KA (1995) Interleukin-2 Deficient Mice: A New Model to Study Autoimmunity and Self‐Tolerance. Immunol Rev 148:35–44. https://doi.org/10.1111/j.1600-065X.1995.tb00092.x
Haura EB, Turkson J, Jove R (2005) Mechanisms of disease: Insights into the emerging role of signal transducers and activators of transcription in cancer. Nat Clin Pract Oncol 2:315–324. https://doi.org/10.1038/NCPONC0195
Yu H, Jove R (2004) The STATs of cancer–new molecular targets come of age. Nat Rev Cancer 4:97–105. https://doi.org/10.1038/NRC1275
Orlova A, Wagner C, De Araujo ED et al (2019) Direct Targeting Options for STAT3 and STAT5 in Cancer. Cancers (Basel) 11:1930. https://doi.org/10.3390/CANCERS11121930
Shi YN, Zhu N, Liu C et al (2017) Wnt5a and its signaling pathway in angiogenesis. Clin Chim Acta 471:263–269. https://doi.org/10.1016/J.CCA.2017.06.017
Torpey N, Maher SE, Bothwell ALM, Pober JS (2004) Interferon α but not interleukin 12 activates STAT4 signaling in human vascular endothelial cells. J Biol Chem 279:26789–26796. https://doi.org/10.1074/jbc.M401517200
Nishimura Y, Nitto T, Inoue T, Node K (2008) IL-13 attenuates vascular tube formation via JAK2-STAT6 pathway. Circ J 72:469–475. https://doi.org/10.1253/circj.72.469
Stephanou A, Latchman DS (2005) Opposing actions of STAT-1 and STAT-3. Growth Factors 23:177–182. https://doi.org/10.1080/08977190500178745
Battle TE, Lynch RA, Frank DA (2006) Signal transducer and activator of transcription 1 activation in endothelial cells is a negative regulator of angiogenesis. Cancer Res 66:3649–3657. https://doi.org/10.1158/0008-5472.CAN-05-3612
Ramnath N, Creaven PJ (2004) Matrix metalloproteinase inhibitors. Curr Oncol Rep 6:96–102. https://doi.org/10.1007/s11912-004-0020-7
Kuwano T, Nakao S, Yamamoto H et al (2004) Cyclooxygenase 2 is a key enzyme for inflammatory cytokine-induced angiogenesis. FASEB J 18:300–310. https://doi.org/10.1096/fj.03-0473com
Oliner J, Min H, Leal J et al (2004) Suppression of angiogenesis and tumor growth by selective inhibition of angiopoietin-2. Cancer Cell 6:507–516. https://doi.org/10.1016/j.ccr.2004.09.030
Andreasen P, Kjøller L, Christensen L, Duffy M (1997) The urokinase-type plasminogen activator system in cancer metastasis: a review. Int J Cancer 72:1–22
Raffaella R, Gioia D, De Andrea M et al (2004) The interferon-inducible IFI16 gene inhibits tube morphogenesis and proliferation of primary, but not HPV16 E6/E7-immortalized human endothelial cells. Exp Cell Res 293:331–345. https://doi.org/10.1016/j.yexcr.2003.10.014
Vargas-Hernández A, Witalisz-Siepracka A, Prchal-Murphy M et al (2020) Human signal transducer and activator of transcription 5b (STAT5b) mutation causes dysregulated human natural killer cell maturation and impaired lytic function. J Allergy Clin Immunol 145:345–357e9. https://doi.org/10.1016/j.jaci.2019.09.016
Yang X, Friedl A (2015) A positive feedback loop between prolactin and stat5 promotes angiogenesis. Adv Exp Med Biol 846:265–280. https://doi.org/10.1007/978-3-319-12114-7_12
Yang X, Qiao D, Meyer K et al (2012) Angiogenesis induced by signal transducer and activator of transcription 5A (STAT5A) is dependent on autocrine activity of proliferin. J Biol Chem 287:6490–6502. https://doi.org/10.1074/jbc.M111.254631
Gao P, Niu N, Wei T et al (2017) The roles of signal transducer and activator of transcription factor 3 in tumor angiogenesis. Oncotarget 8:69139–69161. https://doi.org/10.18632/oncotarget.19932
Pawlus MR, Wang L, Hu CJ (2014) STAT3 and HIF1α cooperatively activate HIF1 target genes in MDA-MB-231 and RCC4 cells. Oncogene 33:1670–1679. https://doi.org/10.1038/onc.2013.115
Chen Z, Zhong CH (2008) STAT3: A critical transcription activator in angiogenesis. Med Res Rev 28:185–200. https://doi.org/10.1002/med.20101
Niu G, Wright KL, Huang M et al (2002) Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene 21:2000–2008. https://doi.org/10.1038/sj.onc.1205260
Leifheit-Nestler M, Conrad G, Heida NM et al (2010) Overexpression of integrin β5 enhances the paracrine properties of circulating angiogenic cells via Src kinase-mediated activation of STAT3. Arterioscler Thromb Vasc Biol 30:1398–1406. https://doi.org/10.1161/ATVBAHA.110.206086
Keller S, Schmidt MHH (2017) EGFR and EGFRvIII promote angiogenesis and cell invasion in glioblastoma: Combination therapies for an effective treatment. Int J Mol Sci 18:1295. https://doi.org/10.3390/ijms18061295
Xie TX, Wei D, Liu M et al (2004) Stat3 activation regulates the expression of matrix metalloproteinase-2 and tumor invasion and metastasis. Oncogene 23:3550–3560. https://doi.org/10.1038/sj.onc.1207383
Zhao M, Gao FH, Wang JY et al (2011) JAK2/STAT3 signaling pathway activation mediates tumor angiogenesis by upregulation of VEGF and bFGF in non-small-cell lung cancer. Lung Cancer 73:366–374. https://doi.org/10.1016/j.lungcan.2011.01.002
Krstić M, Stojanović NM, Stojnev S et al (2019) Interplay between STAT3, cell adhesion molecules and angiogenesis-related parameters in gastric carcinoma. Does STAT3 really have a prognostic value? https://doi.org/10.3390/medicina55060300. Med 55:
Kortylewski M, Yu H (2008) Role of Stat3 in suppressing anti-tumor immunity. Curr Opin Immunol 20:228–233. https://doi.org/10.1016/J.COI.2008.03.010
Chen RY, Yen CJ, Liu YW et al (2020) CPAP promotes angiogenesis and metastasis by enhancing STAT3 activity. Cell Death Differ 27:1259–1273. https://doi.org/10.1038/s41418-019-0413-7
Lee H, Jeong AJ, Ye SK (2019) Highlighted STAT3 as a potential drug target for cancer therapy. BMB Rep 52:415–423. https://doi.org/10.5483/BMBREP.2019.52.7.152
Gu FM, Li QL, Gao Q et al (2011) IL-17 induces AKT-dependent IL-6/JAK2/STAT3 activation and tumor progression in hepatocellular carcinoma. Mol Cancer 10. https://doi.org/10.1186/1476-4598-10-150
Kako F, Gabunia K, Ray M et al (2016) Interleukin-19 induces angiogenesis in the absence of hypoxia by direct and indirect immune mechanisms. Am J Physiol - Cell Physiol 310:C931–C941. https://doi.org/10.1152/ajpcell.00006.2016
Yang X, Lin A, Jiang N et al (2017) Interleukin-6 trans-signalling induces vascular endothelial growth factor synthesis partly via Janus kinases-STAT3 pathway in human mesothelial cells. Nephrology 22:150–158. https://doi.org/10.1111/nep.12746
Jee SH, Chu CY, Chiu HC et al (2004) Interleukin-6 Induced basic fibroblast growth factor-dependent angiogenesis in basal cell carcinoma cell line via JAK/STAT3 and PI3-Kinase/Akt pathways. J Invest Dermatol 123:1169–1175. https://doi.org/10.1111/j.0022-202X.2004.23497.x
Khandelwal A, Bacolla A, Vasquez KM, Jain A (2015) Long non-coding RNA: A new paradigm for lung cancer. Mol Carcinog 54:1235–1251. https://doi.org/10.1002/MC.22362
An T, Liu J, Yang Q et al (2021) Anti-tumor Role of MicroRNA-4782-3p in Epithelial Ovarian Cancer. Cancer Plus 3:10–20. https://doi.org/10.18063/CP.V3I1.294
Berry JM, Einzig S, Krabill KA, Bass JL (1988) Evaluation of coronary artery anatomy in patients with tetralogy of Fallot by two-dimensional echocardiography. Circulation 78:149–156. https://doi.org/10.1161/01.CIR.78.1.149
Sharma U, Barwal TS, Acharya V et al (2020) Long Non-Coding RNAs as Strategic Molecules to Augment the Radiation Therapy in Esophageal Squamous Cell Carcinoma. Int J Mol Sci 21:1–18. https://doi.org/10.3390/IJMS21186787
Malhotra A, Sharma U, Puhan S et al (2019) Stabilization of miRNAs in esophageal cancer contributes to radioresistance and limits efficacy of therapy. Biochimie 156:148–157. https://doi.org/10.1016/j.biochi.2018.10.006
Sharma U, Barwal TS, Khandelwal A et al (2021) LncRNA ZFAS1 inhibits triple-negative breast cancer by targeting STAT3. Biochimie 182:99–107. https://doi.org/10.1016/j.biochi.2020.12.026
Barwal TS, Sharma U, Vasquez KM et al (2020) A panel of circulating long non-coding RNAs as liquid biopsy biomarkers for breast and cervical cancers. Biochimie 176:62–70. https://doi.org/10.1016/j.biochi.2020.06.012
Tamang S, Acharya V, Roy D et al (2019) SNHG12: An LncRNA as a Potential Therapeutic Target and Biomarker for Human Cancer. Front Oncol 9:901. https://doi.org/10.3389/fonc.2019.00901
Sharma U, Barwal TS, Malhotra A et al (2020) Long non-coding RNA TINCR as potential biomarker and therapeutic target for cancer. Life Sci 257:118035. https://doi.org/10.1016/j.lfs.2020.118035
Sharma U, Barwal TS, Acharya V et al (2020) Cancer Susceptibility Candidate 9 (CASC9): A Novel Targetable Long Noncoding RNA in Cancer Treatment. Transl Oncol 13:100774. https://doi.org/10.1016/j.tranon.2020.100774
Liu W, Liang F, Yang G, Xian L (2021) LncRNA LINC01116 sponges miR-93-5p to promote cell invasion and migration in small cell lung cancer. BMC Pulm Med 21:50. https://doi.org/10.1186/s12890-020-01369-3
Hua K, Deng X, Hu J et al (2020) Long noncoding RNA HOST2, working as a competitive endogenous RNA, promotes STAT3-mediated cell proliferation and migration via decoying of let-7b in triple-negative breast cancer. J Exp Clin Cancer Res 39:58. https://doi.org/10.1186/s13046-020-01561-7
Wang Y, Wu S, Zhu X et al (2020) LncRNA-encoded polypeptide ASRPS inhibits triple-negative breast cancer angiogenesis. J Exp Med 217:20190950. https://doi.org/10.1084/jem.20190950
Wang J, Zhou J, Jiang C et al (2019) LNRRIL6, a novel long noncoding RNA, protects colorectal cancer cells by activating the IL-6–STAT3 pathway. Mol Oncol 13:2344–2360. https://doi.org/10.1002/1878-0261.12538
Liu B, Liu Q, Pan S et al (2019) The HOTAIR/miR-214/ST6GAL1 crosstalk modulates colorectal cancer procession through mediating sialylated c-Met via JAK2/STAT3 cascade. J Exp Clin Cancer Res 38:455. https://doi.org/10.1186/s13046-019-1468-5
WANG Y, FU J, LIANG YANGL Z (2020) Long non-coding RNA SNHG20 promotes colorectal cancer cell proliferation, migration and invasion via miR-495/STAT3 axis. Mol Med Rep 23:31. https://doi.org/10.3892/mmr.2020.11669
Zhang L, Ye F, Zuo Z et al (2021) Long noncoding RNA TPT1-AS1 promotes the progression and metastasis of colorectal cancer by upregulating the TPT1-mediated FAK and JAK-STAT3 signalling pathways. Aging 13:3779–3797. https://doi.org/10.18632/aging.202339
Darnell JE (1997) STATs and gene regulation. Sci (80-) 277:1630–1635. https://doi.org/10.1126/science.277.5332.1630
Zheng HC (2017) The molecular mechanisms of chemoresistance in cancers. Oncotarget 8:59950–59964. https://doi.org/10.18632/oncotarget.19048
Tzeng YDT, Liu PF, Li JY et al (2018) Kinome-wide siRNA screening identifies Src-enhanced resistance of chemotherapeutic drugs in triple-negative breast cancer cells. Front Pharmacol 9. https://doi.org/10.3389/fphar.2018.01285
Castellaro AM, Rodriguez-Baili MC, Di Tada CE, Gil GA (2019) Tumor-associated macrophages induce endocrine therapy resistance in ER + breast cancer cells. Cancers (Basel) 11. https://doi.org/10.3390/cancers11020189
Wang T, Fahrmann JF, Lee H et al (2018) JAK/STAT3-Regulated Fatty Acid β-Oxidation Is Critical for Breast Cancer Stem Cell Self-Renewal and Chemoresistance. Cell Metab 27:136–150e5. https://doi.org/10.1016/j.cmet.2017.11.001
Wang S, Yao Y, Yao M et al (2018) Interleukin-22 promotes triple negative breast cancer cells migration and paclitaxel resistance through JAK-STAT3/MAPKs/AKT signaling pathways. Biochem Biophys Res Commun 503:1605–1609. https://doi.org/10.1016/j.bbrc.2018.07.088
Liu C, Xing H, Guo C et al (2019) MiR-124 reversed the doxorubicin resistance of breast cancer stem cells through STAT3/HIF-1 signaling pathways. Cell Cycle 18:2215–2227. https://doi.org/10.1080/15384101.2019.1638182
Cheng CC, Shi LH, Wang XJ et al (2018) Stat3/Oct-4/c-Myc signal circuit for regulating stemness-mediated doxorubicin resistance of triple-negative breast cancer cells and inhibitory effects of WP1066. Int J Oncol 53:339–348. https://doi.org/10.3892/ijo.2018.4399
Kim JY, Kim JC, Lee JY, Park MJ (2018) Oct4 suppresses IR-induced premature senescence in breast cancer cells through STAT3- and NF-κB-mediated IL-24 production. Int J Oncol 53:47–58. https://doi.org/10.3892/ijo.2018.4391
Xiang S, Dauchy RT, Hoffman AE et al (2019) Epigenetic inhibition of the tumor suppressor ARHI by light at night-induced circadian melatonin disruption mediates STAT3-driven paclitaxel resistance in breast cancer. J Pineal Res 67. https://doi.org/10.1111/jpi.12586
Tseng CC, Zhang P, Lee AS (2019) The COOH-Terminal Proline-Rich Region of GRP78 Is a Key Regulator of Its Cell Surface Expression and Viability of Tamoxifen-Resistant Breast Cancer Cells. Neoplasia (United States) 21:837–848. https://doi.org/10.1016/j.neo.2019.05.008
Wang L, Wang Q, Gao M et al (2018) STAT3 activation confers trastuzumab-emtansine (T-DM1) resistance in HER2-positive breast cancer. Cancer Sci 109:3305–3315. https://doi.org/10.1111/cas.13761
Feng F, Zhu X, Wang C et al (2018) Downregulation of hypermethylated in cancer-1 by miR-4532 promotes adriamycin resistance in breast cancer cells. Cancer Cell Int 18. https://doi.org/10.1186/s12935-018-0616-x
Chen D, Ma Y, Li P et al (2019) Piperlongumine induces apoptosis and synergizes with doxorubicin by inhibiting the JAK2-STAT3 pathway in triple-negative breast cancer. Molecules 24. https://doi.org/10.3390/molecules24122338
Dorayappan KDP, Wanner R, Wallbillich JJ et al (2018) Hypoxia-induced exosomes contribute to a more aggressive and chemoresistant ovarian cancer phenotype: A novel mechanism linking STAT3/Rab proteins. Oncogene 37:3806–3821. https://doi.org/10.1038/s41388-018-0189-0
Codony-Servat J, Marín-Aguilera M, Visa L et al (2013) Nuclear factor-kappa B and interleukin-6 related docetaxel resistance in castration-resistant prostate cancer. Prostate 73:512–521. https://doi.org/10.1002/pros.22591
Zemskova M, Sahakian E, Bashkirova S, Lilly M (2008) The PIM1 kinase is a critical component of a survival pathway activated by docetaxel and promotes survival of docetaxel-treated prostate cancer cells. J Biol Chem 283:20635–20644. https://doi.org/10.1074/jbc.M709479200
Puhr M, Hoefer J, Schäfer G et al (2012) Epithelial-to-mesenchymal transition leads to docetaxel resistance in prostate cancer and is mediated by reduced expression of miR-200c and miR-205. Am J Pathol 181:2188–2201. https://doi.org/10.1016/j.ajpath.2012.08.011
Patterson SG, Wei S, Chen X et al (2006) Novel role of Stat1 in the development of docetaxel resistance in prostate tumor cells. Oncogene 25:6113–6122. https://doi.org/10.1038/sj.onc.1209632
Spiotto MT, Chung TDK (2000) STAT3 mediates IL-6-induced neuroendocrine differentiation in prostate cancer cells. Prostate 42:186?195. https://doi.org/10.1002/(SICI)1097-0045(20000215)42:3<186::AID-PROS4>3.0.CO;2-E
Zhou W, Fu XQ, Zhang LL et al (2013) The AKT1/NF-kappaB/Notch1/PTEN axis has an important role in chemoresistance of gastric cancer cells. Cell Death Dis 4:e847–e847. https://doi.org/10.1038/cddis.2013.375
Awasthi N, Liongue C, Ward AC (2021) STAT proteins: a kaleidoscope of canonical and non-canonical functions in immunity and cancer. J Hematol Oncol 14:198. https://doi.org/10.1186/s13045-021-01214-y
Bollrath J, Phesse TJ, von Burstin VA et al (2009) gp130-Mediated Stat3 Activation in Enterocytes Regulates Cell Survival and Cell-Cycle Progression during Colitis-Associated Tumorigenesis. Cancer Cell 15:91–102. https://doi.org/10.1016/j.ccr.2009.01.002
Sun R, Liu Z, Qiu B et al (2019) Annexin10 promotes extrahepatic cholangiocarcinoma metastasis by facilitating EMT via PLA2G4A/PGE2/STAT3 pathway. EBioMedicine 47:142–155. https://doi.org/10.1016/j.ebiom.2019.08.062
Sun C, Bernards R (2014) Feedback and redundancy in receptor tyrosine kinase signaling: Relevance to cancer therapies. Trends Biochem Sci 39:465–474. https://doi.org/10.1016/j.tibs.2014.08.010
Qin JJ, Yan L, Zhang J, Zhang WD (2019) STAT3 as a potential therapeutic target in triple negative breast cancer: A systematic review. J Exp Clin Cancer Res 38:195. https://doi.org/10.1186/s13046-019-1206-z
Kishimoto M, Komine M, Sashikawa-Kimura M et al (2021) Stat3 activation in psoriasis and cancers. https://doi.org/10.3390/diagnostics11101903. Diagnostics 11:1903
Park JS, Kwok SK, Lim MA et al (2014) STA-21, a promising STAT-3 inhibitor that reciprocally regulates Th17 and Treg cells, inhibits osteoclastogenesis in mice and humans and alleviates autoimmune inflammation in an experimental model of rheumatoid arthritis. Arthritis Rheumatol 66:918–929. https://doi.org/10.1002/art.38305
Gu Y, Mohammad IS, Liu Z (2020) Overview of the STAT-3 signaling pathway in cancer and the development of specific inhibitors. Oncol Lett 19:2585–2594. https://doi.org/10.3892/OL.2020.11394
Zhao C, Xiao H, Wu X et al (2015) Rational combination of MEK inhibitor and the STAT3 pathway modulator for the therapy in K-Ras mutated pancreatic and colon cancer cells. Oncotarget 6:14472–14487. https://doi.org/10.18632/oncotarget.3991
Xiao H, Bid HK, Jou D et al (2015) A novel small molecular STAT3 inhibitor, LY5, inhibits cell viability, cell migration, and angiogenesis in medulloblastoma cells. J Biol Chem 290:3418–3429. https://doi.org/10.1074/jbc.M114.616748
Takakura A, Nelson EA, Haque N et al (2011) Pyrimethamine inhibits adult polycystic kidney disease by modulating STAT signaling pathways. Hum Mol Genet 20:4143–4154. https://doi.org/10.1093/hmg/ddr338
Zou S, Tong Q, Liu B et al (2020) Targeting STAT3 in Cancer Immunotherapy. Mol Cancer 19:1–19. https://doi.org/10.1186/S12943-020-01258-7
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
HST and KS designed and written; AI contributed in clinical section; VKG contributed in chemoresistance section; MV contributed in angiogenesis section; US contributed in ncRNA section; AC edited the final paper; MBY contributed in inflammation section; KD and MJ contributed to manuscript revision; AJ contributed in ncRNA section and draft reading.
Corresponding authors
Ethics declarations
Conflict of interest
Authors declared there is no conflict of interest.
Ethical approval
This is a review paper. Therefore, no ethical approval is required.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tuli, H.S., Sak, K., Iqubal, A. et al. STAT signaling as a target for intervention: from cancer inflammation and angiogenesis to non-coding RNAs modulation. Mol Biol Rep 49, 8987–8999 (2022). https://doi.org/10.1007/s11033-022-07399-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07399-w