Abstract
Background
Pigeonpea (Cajanus cajan L.) is a photoperiod-sensitive short-day plant. Understanding the flowering-related genes is critical to developing photoperiod insensitive cultivars.
Methods
The CCT family genes were identified using ‘CCT DOMAIN PROTEIN’ as a keyword and localized on the chromosomes using the BLAST search option available at the LIS database. The centromeric positions were identified through BLAST search using the centromeric repeat sequence of C. cajan as a query against the chromosome-wise FASTA files downloaded from the NCBI database. The CCT family genes were classified based on additional domains and/or CCT domains. The orthologous and phylogenetic relationships were inferred using the OrthoFinder and MEGA 10.1 software, respectively. The CCT family genes′ expression level in photoperiod-sensitive and insensitive genotypes was compared using RNA-seq data and qRT-PCR analysis.
Results
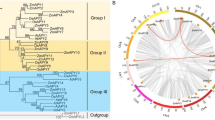
We identified 33 CCT family genes in C. cajan distributed on ten chromosomes and nine genomic scaffolds. They were classified into CMF-type, COL-type, PRR-type, and GTCC- type. The CCT family genes of legumes exhibited an extensive orthologous relationship. Glycine max showed the maximum similarity of CCT family genes with C. cajan. The expression analysis of CCT family genes using photoperiod insensitive (ICP20338) and photoperiod sensitive (MAL3) genotypes of C. cajan demonstrated that CcCCT4 and CcCCT23 are the active CONSTANS in ICP20338. In contrast, only CcCCT23 is active in MAL3.
Conclusion
The CCT family genes in C. cajan vary considerably in structure and domain types. They are maximally similar to soybean’s CCT family genes. The differential photoperiod response of pigeonpea genotypes, ICP20338 and MAL3, is possibly due to the difference in the number and types of active CONSTANS in them.





Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Zhang S, Yang C, Peng J, Sun S, Wang X (2009) GASA5, a regulator of flowering time and stem growth in Arabidopsis thaliana. Plant Mol Biol 69(6):745–759. https://doi.org/10.1007/s11103-009-9452-7
Trevaskis B, Hemming MN, Dennis ES et al (2007) The molecular basis of vernalization induced flowering in cereals. Trends Plant Sci 12:352–357. https://doi.org/10.1016/j.tplants.2007.06.010
Agliassa C, Narayana R, Bertea CM et al (2018) Reduction of the geomagnetic field delays Arabidopsis thaliana flowering time through downregulation of flowering-related genes. Bioelectromagnetics 39:361–374. https://doi.org/10.1002/bem.22123
Song YH, Shim JS, Kinmonth-Schultz HA et al (2015) Photoperiodic flowering: time measurement mechanisms in leaves. Korea Annu Rev Plant Biol 66:441–464. https://doi.org/10.1146/annurev-arplant-043014-1155555
Putterill JJ, Robson F, Lee K et al (1995) The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell 80:847–857. https://doi.org/10.1016/0092-8674(95)90288-0
Corbesier L, Vincent C, Jang S et al (2007) FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 316:1030–1033. https://doi.org/10.1126/science.1141752
Eliezer L, Brian A, Yuval E (2014) Florigen and anti-florigen—a systemic mechanism for coordinating growth and termination in flowering plants. Front Plant Sci 5:465. https://doi.org/10.3389/fpls.2014.00465
Tamaki S, Matsuo S, Wong HL et al (2007) Hd3a Protein is a mobile flowering signal in rice. Science 316:1033–1036. https://doi.org/10.1126/science.1141753
Cheng X, Li G, Tang Y et al (2018) Dissection of genetic regulation of compound inflorescence development in Medicago truncatula. Development 145(3):dev158766. https://doi.org/10.1242/dev.158766
Taoka K, Ohki I, Tsuji H et al (2011) 14–3-3 proteins act as intracellular receptors for rice Hd3a florigen. Nature 476:332–335. https://doi.org/10.1038/nature10272
Golembeski GS, Imaizumi T (2015) Photoperiodic regulation of florigen function in Arabidopsis thaliana. Arabidopsis Book/Am Soc Plant Biol 13:e0178. https://doi.org/10.1199/tab.0178
David KM, Armbruster U, Tama N et al (2006) Arabidopsis GIGANTEA protein is post-transcriptionally regulated by light and dark. FEBS Lett 580:1193–1197. https://doi.org/10.1016/j.febslet.2006.01.016
Niwa Y, Ito S, Nakamichi N et al (2007) Genetic linkages of the circadian clock associated genes TOC1 CCA1 and LHY in the photoperiodic control of flowering time in Arabidopsis thaliana. Plant Cell Physiol 48:925–937. https://doi.org/10.1093/pcp/pcm067
Sawa M, Nusinow DA, Kay SA et al (2007) FKF1 and GIGANTEA complex formation is required for day-length measurement in Arabidopsis. Science 318:261–265. https://doi.org/10.1126/science.1146994
Fornara F, Panigrahi KCS, Gissot L et al (2009) Arabidopsis DOF transcription factors act redundantly to reduce CONSTANS expression and are essential for a photoperiodic flowering response. Dev Cell 17:75–86. https://doi.org/10.1016/j.devcel.2009.06.015
Nakamichi N, Kiba T, Henriques R et al (2010) PSEUDO-RESPONSE REGULATORS 9 7 and 5 are transcriptional repressors in the Arabidopsis circadian clock. Plant Cell 22:594–605. https://doi.org/10.1105/tpc.109.072892
An H, Roussot C, Suarez LP et al (2004) CONSTANS acts in the phloem to regulate a systemic signal that induces photoperiodic flowering of Arabidopsis. Development 131:3615–3626. https://doi.org/10.1242/dev.01231
Khanna R, Kronmiller B, Maszle DR et al (2009) The Arabidopsis B-Box Zinc finger family. Plant Cell 21:3416–3420. https://doi.org/10.1105/tpc.109.069088
Li Y, Xu M (2017) CCT family genes in cereal crops: a current overview. Crop J 5:449–458. https://doi.org/10.1016/j.cj.2017.07.001
Gao H, Jin M, Zheng XM et al (2014) Days to heading-7 a major quantitative locus determining photoperiod sensitivity and regional adaptation in rice. Proc Natl Acad Sci 111:16337–16342. https://doi.org/10.1073/pnas.1418204111
Beales J, Turner A, Griffiths S et al (2007) A pseudo-response regulator is misexpressed in the photoperiod insensitive Ppd-D1a mutant of wheat (Triticum aestivum L.). Theor Appl Genet 115:721–733. https://doi.org/10.1007/s00122-007-0603-4
Fuller DQ, Murphy C, Kingwell-Banham E et al (2019) Cajanus cajan (L.) Millsp. origins and domestication: the South and Southeast Asian archaeobotanical evidence. Genet Resour Crop Evol 66:1175–1188. https://doi.org/10.1007/s10722-019-00774-w
Srivarsha J, Jahagirdar JE, Kumar CVS et al (2018) Performance of parents and hybrids of pigeonpea (Cajanus cajan (L.) Millsp.) in terms of yield and yield contributing characters. Int J Pure App Biosci 6(1):1271–1275
Velez-Colon R, Garrison SA (1998) Growth maturity and flowering of pigeon peas (Cajanus cajan L. Millsp.) at high latitudes. J Agric Univ 73:223–229
Tribhuvan KU, Das A, Srivastava H et al (2020) Identification and characterization of PEBP family genes reveal CcFT8 a probable candidate for photoperiod insensitivity in C. cajan. 3 Biotech 10:194. https://doi.org/10.1007/s13205-020-02180-x
Zhang L, Li Q, Dong H et al (2015) Three CCT domain-containing genes were identified to regulate heading date by candidate gene-based association mapping and transformation in rice. Sci Rep 5:7663. https://doi.org/10.1038/srep07663
Voorrips RE (2002) MapChart: software for the graphical presentation of linkage maps and QTLs. J Hered 93:77–78. https://doi.org/10.1093/jhered/93.1.77
Melters DP, Bradnam KR, Young HA et al (2013) Comparative analysis of tandem repeats from hundreds of species reveals unique insights into centromere evolution. Genome Biol 14:R10. https://doi.org/10.1186/gb-2013-14-1-r10
Kumar S, Stecher G, Li M et al (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–2154. https://doi.org/10.1093/molbev/msy096
Zheng X, Li X, Ge C et al (2017) Characterization of the CCT family and analysis of gene expression in Aegilops tauschii. PLoS ONE 12(12):e0189333. https://doi.org/10.1371/journal.pone.0189333
Cockram J, Thiel T, Steuernagel B et al (2012) Genome dynamics explain the evolution of flowering time CCT domain gene families in the poaceae. PLoS ONE 7(9):e45307. https://doi.org/10.1371/journal.pone.0045307
Shoemaker R, Olson T, Kanazin V (1996) Soybean genome organization: evolution of a legume genome. In: Gustafson JP, Flavell RB (eds) Genomes of plants and animals. Stadler genetics symposia series. Springer, Boston. https://doi.org/10.1007/978-1-4899-0280-1_11
Zahn-Zabal M, Dessimoz C, Glover NM (2020) Identifying orthologs with OMA: a primer. F1000Research 9:27. https://doi.org/10.12688/f1000research.21508.1
Wolfe KH (2001) Yesterday’s polyploids and the mystery of diploidization. Nat Rev Genet 2:333–341. https://doi.org/10.1038/35072009
Nowak MA, Boerlijst MC, Cooke J et al (1997) Evolution of genetic redundancy. Nature 338:167–171. https://doi.org/10.1038/40618
Taverna DM, Goldstein RM (2000) The evolution of duplicated genes considering protein stability constraints. Pacific Symposium on Biocomputing. World Scientific, Singapore, pp 69–80
Wagner A (2000) The role of population size, pleiotropy and fitness effects of mutations in the evolution of overlapping gene functions. Genetics 154:1389–1401
Force A, Lynch M, Pickett FB et al (1999) Preservation of duplicate genes by complementary, degenerative mutations. Genetics 151:1531–1545
Lee C, Yu D, Choi HK et al (2017) Reconstruction of a composite comparative map composed of ten legume genomes. Genes Genom 39(1):111–119. https://doi.org/10.1007/s13258-016-0481-8
Tsuji H, Taoka K (2014) Chapter five-florigen signaling. In: Machida Y, Lin C, Tamanoi F (eds) The enzymes, vol 35. Academic Press, Cambridge, pp 113–144. https://doi.org/10.1016/B978-0-12-801922-1.00005-1
Acknowledgements
We sincerely acknowledge the Director, ICAR-NIPB, New Delhi; Director, ICAR-IARI, New Delhi; Director, ICAR-IIAB, Ranchi; and the Incharge, National Phytotron Facility, ICAR- IARI, New Delhi for providing the necessary facilities to carry out the research work.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
KG and NKS designed the work. KUT, HS, and TK conducted the experiments. BKS, AD, KK, KD and RJ analyzed and interpreted the data and wrote and revised the manuscript critically. All the authors read the manuscript and approved the final version for publication.
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare no competing interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tribhuvan, K.U., Kaila, T., Srivastava, H. et al. Structural and functional analysis of CCT family genes in pigeonpea. Mol Biol Rep 49, 217–226 (2022). https://doi.org/10.1007/s11033-021-06860-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06860-6