Abstract
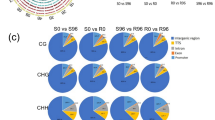
Differential DNA methylation due to Lr28 was examined in susceptible (S) wheat cv. HD2329 and its resistant (R) near isogenic line (NIL) (HD2329+Lr28) using two approaches: methylation sensitive amplified polymorphism (MSAP) and methylated DNA immunoprecipitation (MeDIP). S/R lines each had a large number of hypomethylated genes and relatively fewer hypermethylated genes at 96 hai (hours after inoculation) relative to 0 hbi (hours before inoculation), suggesting activation of many genes during the passage of time (96 hai), although identity of genes may differ in S and R lines. When R NIL was compared with S cultivar, there were many hypermethylated and fewer hypomethylated genes in R NIL relative to S cultivar, suggesting that many genes that are active in S cultivar are silenced in R NIL, both at 0 hbi and at 96 hai. Level of methylation was generally abundant in intergenic regions followed by that in promoters, transcription termination sites (TTSs) and exons/introns. Hypermethylation in promoter and gene body regions was not always associated with inhibition of gene expression and vice-versa, indicating that more than one regulatory mechanisms may control the expression of genes due to pathogen attack in presence and absence of Lr28. MSAP analysis also showed abundance of mCG methylation in S cultivar and that of mCCG methylation in R NIL (at 96 hai), suggesting differences in methylation context in NILs with and without Lr28. The results of the present study improved our understanding of the epigenetic control of leaf rust resistance in wheat.







Similar content being viewed by others
References
Ray DK, Mueller ND, West PC, Foley JA (2013) Yield trends are insufficient to double global crop production by 2050. PLoS ONE 8:e66428
Curtis T, Halford NG (2014) Food security: the challenge of increasing wheat yield and the importance of not comprising food security. Ann Appl Biol 164:354–372
Draz I, Abou-Elseoud MS, Kamara AEM, Alaa-Eldein OA, El-Bebany A (2015) Screening of wheat genotypes for leaf rust resistance along with grain yield. Ann Agric Sci 60:29–39
McIntosh RA, Dubcovsky J, Rogers WJ, Morris C, Xia XC (2017) Catalogue of gene symbols for wheat: 2017 supplement. [Verified 12 April 2018] 36:103–110. https://shigen.nig.ac.jp/wheat/komugi/genes/macgene/supplement2017.pdf
Feuillet C, Travella S, Stein N, Albar L, Nublat A, Keller B (2003) Map-based isolation of the leaf rust disease resistance gene Lr10 from the hexaploidy wheat (Triticum aestivum L.) genome. Proc Natl Acad of Sci USA 100:15253–15258
Huang L, Brooks SA, Li W, Felers JP, Trick HN, Gill BS (2003) Map-based cloning of leaf rust resistance gene Lr21 from the large and polyploid genome of bread wheat. Genetics 164:655–664
Cloutier S, McCallum BD, Loutre C, Banks TW, Wicker T et al (2007) Leaf rust resistance gene Lr1, isolated from bread wheat (Triticum aestivum L.) is a member of the large psr567 gene family. Plant Mol Biol 65:93–106
Krattinger SG, Lagudah ES, Spielmeyer W, Singh RP, Huerta-Espino J, McFadden H et al (2009) A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat. Science 323:1360–1363
Salcedo A, Rutter W, Wang S, Akhunova A, Bolus S, Chao S et al (2017) Variation in the AvrSr35 gene determines Sr35 resistance against wheat stem rust race Ug99. Science 358:1604–1606
Chen J, Upadhayay NM, Ortiz D, Sperschneider J, Bouton C, Breen S et al (2017) Loss of AvrSr50 by somatic exchange in stem rust leads to virulence for Sr50 resistance in wheat. Science 358:1607–1610
Wang J, Hu M, Wang J, Ki J, Han Z, Wang G, Qi Y, Wang HW, Zhou JM, Chai J (2019) Reconstitution and structure of a plant NLR resistosome conferring immunity. Science 364:eaav5870
Horsefield S, Burdett H, Zhang X, Manik MK, Shi Y, Chen J, Qi T et al (2019) NAD+ cleavage activity by animal and plant TIR domains in cell death pathways. Science 365:793–799
Wan L, Essuman K, Anderson RG, Sasaki Y, Monteiro F, Chung EH, Nishimura EO et al (2019) TIR domains of plant immune receptors are NAD+-cleaving enzymes that promote cell death. Science 365:799–803
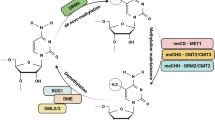
Zhang M, Kimatu JN, Xu K, Liu B (2010) DNA cytosine methylation in plant development. J Genet Genomics 37:1–12
Sha AH, Lin XH, Huang JB, Zhang DP (2005) Analysis of DNA methylation related to rice adult plant resistance to bacterial blight based on methylation-sensitive AFLP (MSAP) analysis. Mol Genet Genomics 273:484–490
Kim MY, Zilberman D (2014) DNA methylation as a system of plant genomic immunity. Trends Plant Sci 19:320–326
Zhang H, Lang Z, Zhu JK (2018) Dynamics and function of DNA methylation in plants. Nat Rev Mol Cel Biol 19:489–506
Paszkowski J, Whitham SA (2001) Gene silencing and DNA methylation processes. Curr Opin Plant Biol 4:123–129
Maunakea AK, Chepelev I, Cui K, Zhao K (2013) Intragenic DNA methylation modulates alternative splicing by recruiting MeCP2 to promote exon recognition. Cell Res 23:1256–1269
Yan X, Dong X, Liu L, Yang Y, Lai J, Guo Y (2016) DNA methylation signature of intergenic region involves in nucleosome remodeler DDM1-mediated repression of aberrant gene transcriptional read-through. J Genet Genomics 43:513–523
Wang W, Quin Q, Sun F, Wang Y, Xu D, Li Z et al (2016) Genome-wide differences in DNA methylation changes in two contrasting rice genotypes in response to drought conditions. Front Plant Sci 7:1675
Lim YC, Li J, Ni Y, Liang Q, Zhang J et al (2017) A complex association between DNA methylation and gene expression in human placenta at first and third trimesters. PLoS ONE 12:e0181155
Reyna-Lopez GE, Simpson J, Ruiz-Herrera J (1997) Differences in DNA methylation patterns are detectable during the dimorphic transition of fungi by amplification of restriction polymorphisms. Mol Gen Genet 253:703–710
Wang M, Qin L, Xie C, Li W, Yuan J, Kong L, Yu W, Xia G, Liu S (2014) Induced and constitutive DNA methylation in a salinity-tolerant wheat introgression line. Plant Cell Physiol 55:1354–1365
Fu SJ, Wang H, Feng LN, Sun Y, Yang WX, Liu DQ (2009) Analysis of methylation sensitive amplified polymorphism in wheat genome under the wheat leaf rust stress. Yi Chuan 31:297–304
Wang D, Zhao J, Bai Y, Ao Y, Guo C (2017) The variation analysis of DNA methylation in wheat carrying gametocidal chromosome 3C from Aegilops triuncialis. Int J Mol Sci 18:1738
Gardiner LJ, Tulloch MQ, Olohan L, Price J, Hall N, Hall A (2015) A genome-wide survey of DNA methylation in hexaploid wheat. Genome Biol 16:273
Kumar S, Beena AS, Awana M, Singh A (2017) Salt-induced tissue specific cytosine methylation downregulates expression of HKT genes in contrasting wheat (Triticum aestivum) genotypes. DNA Cell Biol 36:283–294
Olohan L, Gardiner LJ, Lucaci A, Steuernagel B, Wulff B, Kenny J et al (2018) A modified sequence capture approach allowing standard and methylation analyses of the same enriched genomic DNA sample. BMC Genomics 19:250
Pan L, Liu X, Wang Z (2012) Comparative DNA methylation analysis of powdery mildew susceptible and resistant near-isogenic lines in common wheat. Life Sci J 10:2073–2083
Sherman JD, Talbert LE (2002) Vernalisation induced changes of the DNA methylation pattern in winter wheat. Genome 45:253–260
Meng FR, Li YC, Yin J, Liu H, Chen XJ, Ni ZF et al (2012) Analysis of DNA methylation during the germination of wheat seeds. Biol Plant 56:269–275
Sun H, Guo Z, Gao L, Zhao G, Zhang W, Zhou R et al (2014) DNA methylation pattern of photoperiod-B1 is associated with photoperiod insensitivity in wheat (Triticum aestivum). New Phytol 204:682–692
Gao L, Diarso M, Zhang A, Zhang H, Dong Y, Liu L, Lv Z, Liu B (2016) Heritable alteration of DNA methylation induced by whole-chromosome aneuploidy in wheat. New Phytol 209:364–375
Yong Z, Zhao HL, Cheng L, ZuJun Y, JinHua P, JiangPing Z et al (2008) Analysis of DNA methylation variation in wheat genetic background after alien chromatin introduction based on methylation-sensitive amplification polymorphism. Chin Sci Bull 53:58–69
Shaked H, Kashkush K, Ozkan H, Feldman M, Levy AA (2001) Sequence elimination and cytosine methylation are rapid and reproducible responses of the genome to wide hybridization and allopolyploidy in wheat. Plant Cell 13:1749–1759
Levy AA, Feldman M (2004) Genetic and epigenetic reprogramming of the wheat genome upon allopolyploidization. Biol J Linn Soc 82:607–613
Bhardwaj SC, Prashar M, Jain SK, Kumar S, Datta D (2010) Virulence of Puccinia triticina on Lr28 in wheat and its evolutionary relation to prevalent pathotypes in India. Cereal Res Commun 38:83–89
Sharma C, Saripalli G, Kumar S, Gautam T, Kumar A, Rani S et al (2018a) A study of transcriptome in leaf rust infected bread wheat involving seedling resistance gene Lr28. Funct Plant Biol 45:1046–1064
Dhariwal R, Vyas S, Govindraj RB, Jha SK, Khurana JP, Tyagi AK, Prabhu KV, Balyan HS, Gupta PK (2011) Analysis of differentially expressed genes in leaf rust infected bread wheat involving seedling resistance gene Lr28. Funct Plant Biol 38:479–492
Singh D, Kumar D, Satapathy L, Pathak J, Chandra S, Riaz A, Bhaganangre G, Dhariwal R, Kumar M, Prabhu KV, Balyan HS, Gupta PK, Mukhopadhyay K (2017) Insights of Lr28 mediated wheat leaf rust resistance: transcriptomic approach. Gene 637:72–89
Chandra S, Singh D, Pathak J, Kumari S, Kumar M, Poddar R, Balyan HS, Gupta PK, Prabhu KV, Mukhopadhyay K (2016) De novo assembled wheat transcriptomes delineate differentially expressed host genes in response to leaf rust infection. PLoS ONE 11:e0148453
Satapathy L, Singh D, Ranjan P, Kumar D, Kumar M, Prabhu KV, Mukhopadhyay K (2014) Transcriptome-wide analysis of WRKY transcription factors in wheat and their leaf rust responsive expression profiling. Mol Genet Genomics 289:1289–1306
Sharma C, Kumar S, Saripalli G, Jain N, Raghuvanshi S, Sharma JB, Prabhu KV, Balyan HS, Gupta PK (2018b) H3K4/K9 acetylatatin and Lr28-mediated expression of six leaf rust responsive genes in wheat (Triticum aestivum). Mol Genet Genomics 94:227–241
Riley R, Chapman V, Johnson R (1968) Introduction of yellow rust resistance of Aegilops comosa into wheat by genetically induced homoeologous recombination. Nature 217:383–384
Friebe B, Jiang J, Raupp W, McIntosh RA, Gill BS (1996) Characterisation of wheat-alien translocations conferring resistance to disease and pests: current status. Euphytica 91:59–87
Tomar SMS, Menon MK (1998) Adult plant response to near-isogenic lines and stocks of wheat carrying specific Lr genes against leaf rust. Indian Phytopathol 51:61–67
Naik S, Gill KS, Prakasa Rao VS, Gupta VS, Tamhankar SA, Pujar S, Gill BS, Ranjekar PK (1998) Identification of a STS marker linked to the Aegilops speltoides-derived leaf rust resistance gene Lr28 in wheat. Theor Appl Genet 97:535–540
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Perez-Figueroa (2013) Msap: a tool for the statistical analysis of methylation-sensitive amplified polymorphism data. Mol Ecol Resour 13:522–527
Fulnecek J, Kovarik A (2014) How to interpret methylation sensitive amplified polymorphism (MSAP) profiles? BMC Genet 15:2
Li N, Ye M, Li Y, Yan Z, Butcher LM, Sun J, Han X, Chen Q, Zang X, Wang J (2010) Whole genome DNA methylation based on high throughput DNA technology. Methods 52:203–212
Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE et al (2008) Model-based analysis of ChIP-Seq (MACS). Genome Biol 9:R137
Lienhard M, Grimm C, Morkel M, Herwig R, Chavez L (2014) MEDIPS: genome-wide differential coverage analysis of sequencing data derived from DNA enrichment experiments. Bioinformatics 30:284–286
Hu J, Chen X, Zhang H, Ding Y (2015) Genome-wide analysis of DNA methylation in photoperiod- and thermo-sensitive male sterile rice Peiai 64S. BMC Genomics 16:102
Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P et al (2010) Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell 38:576–589
Conesa A, Gotz S (2008) Blast2GO: a comprehensive suite for functional analysis in plant genomics. Int J Plant Genomics 2008:619832
Kohany O, Gentles AJ, Hankus L, Jurka J (2006) Annotation, submission and screening of repetitive elements in Repbase: RepbaseSubmitter and Censor. BMC Bioinform 7:47
Livak KJ, Schmittgen (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25:402–408
Wada Y, Miyamoto K, Kusano T, Sano H (2004) Association between up-regulation of stress-responsive genes and hypomethylation of genomic DNA in tobacco plants. Mol Genet Genomics 271:658–666
Zhang YU, Harris CJ, Liu Q, Liu W, Ausin I et al (2018) Large scale comparitive epigenomics reveals hierarchial regulation of non-CG methylation in Arabidopsis. Proc Natl Acad Sci USA 115:E1069–E1074
Garg R, Chevala VVSN, Shankar R, Jain M (2015) Divergent DNA methylation patterns associated with gene expression in rice cultivars with contrasting drought and salinity stress response. Sci Rep 5:14922
Zhong L, Xu Y, Wang J (2009) DNA methylation changes induced by salt stress in wheat Triticum aestivum. Afr J Biotechnol 22:6201–6207
He S, Xu W, Li F, Wang Y, Liu A (2017) Intraspecific DNA methylation polymorphism in the non-edible oilseed plant castor bean. Plant Divers 39:300–307
Karan R, DeLeon T, Biradar H, Subudhi PK (2012) Salt stress induced variation in DNA methylation pattern and its influence on gene expression in contrasting rice genotypes. PLoS ONE 7:e40203
Marconi G, Pace R, Traini A, Raggi L, Lutts S et al (2013) Use of MSAP markers to analyse the effects of salt stress on DNA methylation in rapeseed (Brassica napus var. oleifera). PLoS ONE 8:e75597
Laird M (2010) Principles and applications of genome wide DNA methylation analysis. Nat Rev Genet 11:191–203
Dowen RH, Pelizzola M, Schmitz RJ, Lister R, Dowen JM, Nery JR, Dixon JE, Ecker JR (2012) Widespread DNA methylation in response to biotic stress. Proc Natl Acad Sci USA 109:E2183:E191
Takuno S, Gout BS (2012) Body methylated genes in Arabidopsis thaliana are functionally important and evolve slowly. Mol Biol Evol 219:227
Hollister JD, Gaut BS (2009) Epigenetic silencing of transposable elements: a trade-off between reduced transposition and deleterious effects on neighbouring gene expression. Genome Res 19:1419–1428
Sun L, Miao X, Cui J, Deng J, Wang X, Wang Y et al (2018) Genome-wide high-resolution mapping of DNA methylation identifies epigenetic variation across different salt stress in maize (Zea mays L.) Euphytica 214:25
Curradi M, Izzo A, Badaracco G, Landsberger N (2002) Molecular mechanisms of gene silencing mediated by DNA methylation. Mol Cell Biol 22:3157–3173
Mette MF, Aufsatz W, Winden J, Matzke MA, Matzke AJM (2000) Transcriptional silencing and promoter methylation triggered by double-stranded RNA. EMBO J 19:5194–5201
Liang D, Zhang D, Wu H, Huang C, Shuai P et al (2014) Single-base-resolution methylomes of Populus trichocarpa reveal the association between DNA methylation and drought stress. BMC Genet 15:S9
Xu J, Zhou S, Gong X, Song Y, van Nocker S, Ma F, Guan Q (2018) Single base methylome analysis reveals dynamic epigenomic differences associated with water deficit in apple. Plant Biotechnol J 16:672–687
Deleris A, Halter T, Navarro L (2018) DNA methylation and demethylation in plant immunity. Annu Rev Phyto Pathol 54:579–603
Kumar AA, Raghavaiyya P (2004) Effect of the leaf rust resistance gene Lr28 on grain yield and bread making quality of wheat. Plant Breed 123:35–38
Pavet V, Quintero C, Cecchini NM, Rosa AL, Alvarez ME (2006) Arabidopsis displays centromeric DNA hypomethylation and cytological alterations of heterochromatin upon attack by Pseudomonas syringae. Mol Plant Microbe Interact 19:577–587
López Sánchez A, Stassen JH, Furci L, Smith LM, Ton J (2016) The role of DNA (de)methylation in immune responsiveness of Arabidopsis. Plant J 88:361–374
Shi-wen Wu Hong-wei, Zai-dong W, Ling-rang Y K (2017) Expression comparisons of pathogenesis-related (pr) genes in wheat in response to infection/infestation by Fusarium, ydv aphid-transmitted and hessian fly. J Integr Agric 13:926–936
Borges AF, Ferreira RB, Monteiro S (2013) Transcriptomic changes following the compatible interaction Vitis viniferae-Erysiphe necator. Paving the way towards an enantioselective role in plant defence modulation. Plant Physiol Biochem 68:71–80
Liao Y, Liu S, Jiang Y, Hu C et al (2017) Genome-wide analysis and environmental response profiling of dirigent family genes in rice (Oryza sativa). Genes Genom 39:47–62
Paniagua C, Bilkova A, Jackson P, Dabravolski S et al (2017) Dirigent proteins in plants: modulating cell wall metabolism during abiotic and biotic stress exposure. J Exp Bot 68:3287–3301
Kim KC, Fan B, Chen Z (2006) Pathogen-induced Arabidopsis WRKY7 is a transcriptional repressor and enhances plant susceptibility to Pseudomonas syringae. Plant Physiol 142:1180–1192
Laloi C, Mestres-Ortega D, Marco Y, Meyer Y, Reichheld JP (2004) The Arabidopsis cytosolic thioredoxin h5 gene induction by oxidative stress and its W-box-mediated response to pathogen elicitor. Plant Physiol 134:1006–1016
Perez CM, Spoel SH (2019) Thioredoxin mediated redox signalling in plant immunity. Plant Sci 279:27–33
Shumayla SS, Kumar R, Mendu V, Singh K, Upadhyay SK (2016) Genomic dissection and expression profiling revealed functional divergence in Triticum aestivum leucine rich repeat receptor like kinases (TaLRRKs). Front Plant Sci 7:1374
Qu LJ, Chen J, Liu M et al (2003) Molecular cloning and functional analysis of a novel type of Bowman-Birk inhibitor gene family in rice. Plant Physiol 133:560–570
Piisila M, Keceli MA, Brader G, Jakobson L, Joesaar I, Sipari N, Kollist H, Palva ET, Kariola T (2015) The F-box protein MAX2 contributes to resistance to bacterial phytopathogens in Arabidopsis thaliana. BMC Plant Biol 15:53
Zhou J, Rodriguez-Zas S, Aldea M, Li M, Zhu J, Gonzalez DO, Vodkin LO, DeLucia E, Clough SJ (2005) Expression profiling soybean response to Pseudomonas syringae reveals new defense-related genes and rapid HR-specific downregulation of photosynthesis. Mol Plant Microbe Interact 18:1161–1174
Xin M, Wang X, Peng H, Yao Y, Xie C, Han Y, Ni Z, Sun Q (2012) Transcriptome comparison of susceptible and resistant wheat in response to powdery mildew infection. Genom Proteom Bioin 10:94–106
Delteil A, Gobbato E, Cayrol B, Estevan J, Michel-Romiti C, Dievart A, Kroj T, Morel JB (2016) Several wall-associated kinases participate positively and negatively in basal defense against rice blast fungus. BMC Plant Biol 16:17
Yu Y, Yang D, Zhou S, Gu J, Wang F, Dong J, Huag R (2017) The ethylene response factor OsERF109 negatively affects ethylene biosynthesis and drought tolerance in rice. Protoplasma 254:401–408
Zhang H, Hing Y, Huang L, Li D, Song F (2016) Arabidopsis AtERF014 acts as a dual regulator that differentially modulates immunity against Pseudomonas syringae pv. Sci Rep 6:30251
Ahmed SA, Liu P, Xue Q, Ji C, Qi T, Guo J, Guo J, Kang Z (2017) TaDIR1-2 a wheat ortholog of lipid transfer protein AtDIR1 contributes to negative regulation of wheat rust resistance against Puccinia striiformis f.sp.tritici. Front Plant Sci 8:521
Gullner G, Komives T, Kiraly L, Schroder P (2018) Glutathione S-transferase enzymes in plant-pathogen interactions. Front Plant Sci 9:1836
Krattinger SG, Lagudah ES, Wicker T, Risk JM, Ashton AR, Selter LL, Matsumoto T, Keller B (2011) Lr34 multi-pathogen resistance ABC transporter: molecular analysis of homoeologous and orthologous genes in hexaploid wheat and other grass species. Plant J 65:392–403
Pan Y, Liu Z, Rocheleau H, Fauteux F, Wang Y, McCartney C, Ouellet T (2018) Transcriptome dynamics associated with resistance and susceptibility against fusarium head blight in four wheat genotypes. BMC Genomics 19:642
Rojas CM, Senthil-Kumar M, Tzin V, Mysore KS (2014) Regulation of primary plant metabolism during plant-pathogen interactions and its contribution to plant defense. Front Plant Sci 5:17
Amaral MN, Arge LWP, Benitez LC, Danielowski R (2016) Comparative transcriptomics of rice plants under cold, iron and salt stresses. Funct Integr Genomics 16:567–579
Kumar S, Trivedi PK (2018) Glutathione S-Transferases: Role in combating abiotic stresses including arsenic detoxification in plants. Front Plant Sci 9:751
Hake S, Smith HM, Holtan H, Magnani E, Mele G, Ramirez J (2004) The role of knox genes in plant development. Ann Rev Cell Dev Biol 20:125–151
Muthusamy SK, Dalal M, Chinnusamy V, Bansal CK (2016) Differential regulation of genes coding for organallel and cytosolic ClpATPases under biotic and abiotic stresses in wheat. Front Plant Sci 7:929
Pulido P, Llamas E, Rodriguez-Concepcion M (2017) Both Hsp70 chaperone and Clp protease plastidial systems are required for protection against oxidative stress. Plant Signal Behav 12:e1290039
Kovacs V, Pal M, Vida G, Szalai G, Janda T (2011) Effect of powdery mildew infection on the antioxidant enzyme activities in different lines of Thatcher based wheat. Acta Biol Szeged 55:99–100
Sharma R, Sahoo A, Devendran R, Jain M (2014) Over-expression of a rice tau class glutathione s-transferase gene improves tolerance to salinity and oxidative stresses in Arabidopsis. PLoS ONE 9:e92900
Zhou SM, Kong XZ, Kang HH, Sun XD, Wang W (2015) The involvement of wheat F-box protein gene TaFba1 in the oxidative stress tolerance of plants. PLoS ONE 10:e0122117
Kong X, Zhou S, Yin S, Zhao Z, Han Y, Wang W (2016) Stress inducible expression of an F-box gene TaFBA1 from wheat enhanced the drought tolerance in transgenic tobacco plants without impacting plant growth and development. Front Plant Sci 7:1295
Li Q, Wang W, Wang W, Zhang G, Liu Y, Wang Y, Wang W (2018) Wheat F-box protein gene TaFBA1 is involved in plant tolerance to heat stress. Front Plant Sci 9:521
Powell JJ, Carere J, Fitzgerald TL, Stiller J, Covarelli L, Xu Q et al (2017) The Fusraium crown rot pathogen pseudo graminearum triggers a suite of transcriptional and metabolic changes in bread wheat. Ann Bot 119:853–867
Chen X, Chen H, Yuan JS, Kollner TG, Chen Y, Guo Y et al (2018) The rice terpene synthase gene OsTPS19 functions as an (S)-limonene synthase in planta, and its overexpression leads to enhanced resistance to the blast fungus Magnaporthe oryzae. Plant Biotechnol J 16:1778–1787
Yadav IS, Sharma A, Kaur S, Nahar N, Bhardwaj SC, Sharma TR, Chhuneja P (2016) Comparative temporal transcriptome profiling of wheat near isogenic line carrying Lr57 under compatible and incompatible interactions. Front Plant Sci 7: 1943
Cao C, Xing L, Wang X, Yang X, Wang W, Sun Y et al (2011) Serine/threonine kinase gene Stpk-V, a key member of powdery mildew resistance gene Pm21, confers powdery mildew resistance in wheat. Proc Natl Acad Sci USA 108:7727–7732
Liu X, Yang L, Zhou X, Zhou M, Lu Y, Ma L, Ma H, Zhang Z (2013) Transgenic wheat expressing Thinopyrum intermedium MYB transcription factor TiMYB2R-1shows enhanced resistance to the take-all disease. J Exp Bot 64:2243–2253
Acknowledgements
Financial assistance from the Department of Biotechnology (Grant Number BT/PR3337/AGR/2/819/2011), Government of India for carrying out this study is gratefully acknowledged. GS, CK, TG and KS were each awarded JRF/SRF/RA by the Department of Biotechnology, Government of India. PKG and HSB were each awarded the position of Senior Scientist by Indian National Science Academy, New Delhi. PKG was also awarded a National Academy of Sciences India (NASI) Senior Scientist Platinum Jubilee Fellowship during the tenure of this research work. Facilities available in DBT-sponsored Bioinformatics Infrastructure Facility (BIF) were also utilized for this work.
Author information
Authors and Affiliations
Contributions
PKG and HSB conceived the experiment. GS with the help of CS, KS and TG conducted data analysis. GS and CS also prepared the first draft of the manuscript which was edited and finalised by PKG, HSB and PKS. PP provided inoculum for leaf rust pathotype 77-5 and NJ, KVP and JBS raised and provided the seedlings tissues. JKR assisted GS and CS in conducting MSAP analysis.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that there is no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Saripalli, G., Sharma, C., Gautam, T. et al. Complex relationship between DNA methylation and gene expression due to Lr28 in wheat-leaf rust pathosystem. Mol Biol Rep 47, 1339–1360 (2020). https://doi.org/10.1007/s11033-019-05236-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-05236-1