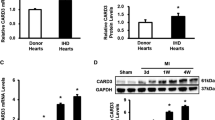
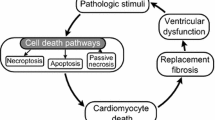
Abstract
The pathophysiological basis of heart failure is cardiac remodeling, a process that comprises structural and functional changes including cardiomyocyte proliferation, hypertrophy, necrosis, apoptosis, autophagy, interstitial fibrosis, contractile dysfunction and ventricular dilatation. Accumulating evidence demonstrate that tumor necrosis factor-like weak inducer of apoptosis (TWEAK) is involved in the process by binding its receptor fibroblast growth factor-inducible molecule 14 (Fn14). In this review, we will discuss the potential role of the TWEAK/Fn14 axis in cardiac remodeling, elucidate its possible mechanisms and explore new therapeutic targets for heart failure.

Similar content being viewed by others
References
Hunt SA, Baker DW, Chin MH et al (2001) ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the International Society for Heart and Lung Transplantation; endorsed by the Heart Failure Society of America. Circulation 104:2996–3007
Schocken DD, Benjamin EJ, Fonarow GC et al (2008) Prevention of heart failure: a scientific statement from the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation 117:2544–2565
Cohn JN, Ferrari R, Sharpe N (2000) Cardiac remodeling—concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an international forum on cardiac remodeling. J Am Coll Cardiol 35:569–582
Leri A, Kajstura J, Anversa P (2002) Myocyte proliferation and ventricular remodeling. J Card Fail 8:S518–S525
Anversa P, Capasso JM (1991) Cardiac hypertrophy and ventricular remodeling. Lab Invest 64:441–445
Tan LB, Jalil JE, Pick R et al (1991) Cardiac myocyte necrosis induced by angiotensin II. Circ Res 69:1185–1195
Sharov VG, Sabbah HN, Shimoyama H et al (1996) Evidence of cardiocyte apoptosis in myocardium of dogs with chronic heart failure. Am J Pathol 148:141–149
Teiger E, Than VD, Richard L et al (1996) Apoptosis in pressure overload-induced heart hypertrophy in the rat. J Clin Invest 97:2891–2897
Olivetti G, Abbi R, Quaini F et al (1997) Apoptosis in the failing human heart. N Engl J Med 336:1131–1141
Miyata S, Takemura G, Kawase Y et al (2006) Autophagic cardiomyocyte death in cardiomyopathic hamsters and its prevention by granulocyte colony-stimulating factor. Am J Pathol 168:386–397
Gurusamy N, Das DK (2009) Autophagy, redox signaling, and ventricular remodeling. Antioxid Redox Signal 11:1975–1988
Ferdous A, Battiprolu PK, Ni YG et al (2010) FoxO, autophagy, and cardiac remodeling. J Cardiovasc Transl Res 3:355–364
Vigliano CA, Cabeza Meckert PM, Diez M et al (2011) Cardiomyocyte hypertrophy, oncosis, and autophagic vacuolization predict mortality in idiopathic dilated cardiomyopathy with advanced heart failure. J Am Coll Cardiol 57:1523–1531
Chen W, Frangogiannis NG (2010) The role of inflammatory and fibrogenic pathways in heart failure associated with aging. Heart Fail Rev 15:415–422
Gong K, Chen YF, Li P et al (2011) Transforming growth factor-beta inhibits myocardial PPARgamma expression in pressure overload-induced cardiac fibrosis and remodeling in mice. J Hypertens 29:1810–1819
Weber KT, Pick R, Silver MA et al (1990) Fibrillar collagen and remodeling of dilated canine left ventricle. Circulation 82:1387–1401
Villarreal FJ, Kim NN, Ungab GD et al (1993) Identification of functional angiotensin II receptors on rat cardiac fibroblasts. Circulation 88:2849–2861
Maekawa Y, Anzai T, Yoshikawa T et al (2002) Prognostic significance of peripheral monocytosis after reperfused acute myocardial infarction: a possible role for left ventricular remodeling. J Am Coll Cardiol 39:241–246
Wrigley BJ, Lip GY, Shantsila E (2011) The role of monocytes and inflammation in the pathophysiology of heart failure. Eur J Heart Fail 13:1161–1171
Moriwaki H, Stempien-Otero A, Kremen M et al (2004) Overexpression of urokinase by macrophages or deficiency of plasminogen activator inhibitor type 1 causes cardiac fibrosis in mice. Circ Res 95:637–644
Kuwahara F, Kai H, Tokuda K et al (2004) Hypertensive myocardial fibrosis and diastolic dysfunction: another model of inflammation? Hypertension 43:739–745
Yu Q, Horak K, Larson DF (2006) Role of T lymphocytes in hypertension-induced cardiac extracellular matrix remodeling. Hypertension 48:98–104
Yu Q, Watson RR, Marchalonis JJ et al (2005) A role for T lymphocytes in mediating cardiac diastolic function. Am J Physiol Heart Circ Physiol 289:H643–H651
Sun M, Chen M, Dawood F et al (2007) Tumor necrosis factor-alpha mediates cardiac remodeling and ventricular dysfunction after pressure overload state. Circulation 115:1398–1407
Burkly LC, Michaelson JS, Hahm K et al (2007) TWEAKing tissue remodeling by a multifunctional cytokine: role of TWEAK/Fn14 pathway in health and disease. Cytokine 40:1–16
Nakajima H, Yanase N, Oshima K et al (2003) Enhanced expression of the apoptosis inducing ligand TRAIL in mononuclear cells after myocardial infarction. Jpn Heart J 44:833–844
Brown NJ (2008) Aldosterone and vascular inflammation. Hypertension 51:161–167
Yu XW, Chen Q, Kennedy RH et al (2005) Inhibition of sarcoplasmic reticular function by chronic interleukin-6 exposure via iNOS in adult ventricular myocytes. J Physiol 566:327–340
Nonaka-Sarukawa M, Okada T, Ito T et al (2008) Adeno-associated virus vector-mediated systemic interleukin-10 expression ameliorates hypertensive organ damage in dahl salt-sensitive rats. J Gene Med 10:368–374
Parissis JT, Farmakis D, Nikolaou M et al (2009) Plasma B-type natriuretic peptide and anti-inflammatory cytokine interleukin-10 levels predict adverse clinical outcome in chronic heart failure patients with depressive symptoms: a 1-year follow-up study. Eur J Heart Fail 11:967–972
Pulkki KJ (1997) Cytokines and cardiomyocyte death. Ann Med 29:339–343
Yin WH, Chen JW, Jen HL et al (2003) The prognostic value of circulating soluble cell adhesion molecules in patients with chronic congestive heart failure. Eur J Heart Fail 5:507–516
Parissis JT, Karavidas A, Bistola V et al (2008) Effects of levosimendan on flow-mediated vasodilation and soluble adhesion molecules in patients with advanced chronic heart failure. Atherosclerosis 197:278–282
Ahmed MS, von Lueder TG, Oie E et al (2005) Induction of myocardial connective tissue growth factor in pacing-induced heart failure in pigs. Acta Physiol Scand 184:27–36
Duisters RF, Tijsen AJ, Schroen B et al (2009) miR-133 and miR-30 regulate connective tissue growth factor: implications for a role of microRNAs in myocardial matrix remodeling. Circ Res 104:170–178 176p following 178
Copaja Soto M, Valenzuela R, Saldana A et al (2008) Early expression of monocyte chemoattractant protein-1 correlates with the onset of isoproterenol-induced cardiac fibrosis in rats with distinct angiotensin-converting enzyme polymorphism. J Renin Angiotensin Aldosterone Syst 9:154–162
Kohno T, Anzai T, Naito K et al (2008) Angiotensin-receptor blockade reduces border zone myocardial monocyte chemoattractant protein-1 expression and macrophage infiltration in post-infarction ventricular remodeling. Circ J 72:1685–1692
Frangogiannis NG, Dewald O, Xia Y et al (2007) Critical role of monocyte chemoattractant protein-1/CC chemokine ligand 2 in the pathogenesis of ischemic cardiomyopathy. Circulation 115:584–592
Morimoto H, Takahashi M, Izawa A et al (2006) Cardiac overexpression of monocyte chemoattractant protein-1 in transgenic mice prevents cardiac dysfunction and remodeling after myocardial infarction. Circ Res 99:891–899
Weir RA, Murphy CA, Petrie CJ et al (2010) Monocyte chemoattractant protein-1: a dichotomous role in cardiac remodeling following acute myocardial infarction in man? Cytokine 50:158–162
Hayashidani S, Tsutsui H, Shiomi T et al (2003) Anti-monocyte chemoattractant protein-1 gene therapy attenuates left ventricular remodeling and failure after experimental myocardial infarction. Circulation 108:2134–2140
Dobaczewski M, Chen W, Frangogiannis NG (2011) Transforming growth factor (TGF)-beta signaling in cardiac remodeling. J Mol Cell Cardiol 51:600–606
Winkles JA (2008) The TWEAK-Fn14 cytokine-receptor axis: discovery, biology and therapeutic targeting. Nat Rev Drug Discov 7:411–425
Brown SA, Richards CM, Hanscom HN et al (2003) The Fn14 cytoplasmic tail binds tumour-necrosis-factor-receptor-associated factors 1, 2, 3 and 5 and mediates nuclear factor-kappaB activation. Biochem J 371:395–403
Saitoh T, Nakayama M, Nakano H et al (2003) TWEAK induces NF-kappaB2 p100 processing and long lasting NF-kappaB activation. J Biol Chem 278:36005–36012
Ando T, Ichikawa J, Wako M et al (2006) TWEAK/Fn14 interaction regulates RANTES production, BMP-2-induced differentiation, and RANKL expression in mouse osteoblastic MC3T3-E1 cells. Arthritis Res Ther 8:R146
Richter B, Rychli K, Hohensinner PJ et al (2010) Differences in the predictive value of tumor necrosis factor-like weak inducer of apoptosis (TWEAK) in advanced ischemic and non-ischemic heart failure. Atherosclerosis 213:545–548
Chorianopoulos E, Rosenberg M, Zugck C et al (2009) Decreased soluble TWEAK levels predict an adverse prognosis in patients with chronic stable heart failure. Eur J Heart Fail 11:1050–1056
Chorianopoulos E, Jarr K, Steen H et al (2010) Soluble TWEAK is markedly upregulated in patients with ST-elevation myocardial infarction and related to an adverse short-term outcome. Atherosclerosis 211:322–326
Filusch A, Zelniker T, Baumgartner C et al (2011) Soluble TWEAK predicts hemodynamic impairment and functional capacity in patients with pulmonary arterial hypertension. Clin Res Cardiol 100:879–885
Martin-Ventura JL, Lindholt JS, Moreno JA et al (2011) Soluble TWEAK plasma levels predict expansion of human abdominal aortic aneurysms. Atherosclerosis 214:486–489
Moreno JA, Dejouvencel T, Labreuche J et al (2010) Peripheral artery disease is associated with a high CD163/TWEAK plasma ratio. Arterioscler Thromb Vasc Biol 30:1253–1262
Karadurmus N, Tapan S, Cakar M et al (2012) Lower plasma soluble TWEAK concentration in patients with newly diagnosed hypertension. Clin Invest Med 35:E20–E26
Lu SX, Ren MY, Wei FT et al (2010) Role of Fn14 in myocardial fibrosis in spontaneous hypertension rats and the intervenient role of perindopril. J Shandong Univ (Health Sci) 48:38–43 (in Chinese)
Chicheportiche Y, Bourdon PR, Xu H et al (1997) TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J Biol Chem 272:32401–32410
Wiley SR, Cassiano L, Lofton T et al (2001) A novel TNF receptor family member binds TWEAK and is implicated in angiogenesis. Immunity 15:837–846
Feng SL, Guo Y, Factor VM et al (2000) The Fn14 immediate-early response gene is induced during liver regeneration and highly expressed in both human and murine hepatocellular carcinomas. Am J Pathol 156:1253–1261
Wiley SR, Winkles JA (2003) TWEAK, a member of the TNF superfamily, is a multifunctional cytokine that binds the TWEAKR/Fn14 receptor. Cytokine Growth Factor Rev 14:241–249
Mustonen E, Sakkinen H, Tokola H et al (2010) Tumour necrosis factor-like weak inducer of apoptosis (TWEAK) and its receptor Fn14 during cardiac remodelling in rats. Acta Physiol (Oxf) 199:11–22
Jain M, Jakubowski A, Cui L et al (2009) A novel role for tumor necrosis factor-like weak inducer of apoptosis (TWEAK) in the development of cardiac dysfunction and failure. Circulation 119:2058–2068
Chorianopoulos E, Heger T, Lutz M et al (2010) FGF-inducible 14-kDa protein (Fn14) is regulated via the RhoA/ROCK kinase pathway in cardiomyocytes and mediates nuclear factor-kappaB activation by TWEAK. Basic Res Cardiol 105:301–313
Novoyatleva T, Diehl F, van Amerongen MJ et al (2010) TWEAK is a positive regulator of cardiomyocyte proliferation. Cardiovasc Res 85:681–690
Munoz-Garcia B, Martin-Ventura JL, Martinez E et al (2006) Fn14 is upregulated in cytokine-stimulated vascular smooth muscle cells and is expressed in human carotid atherosclerotic plaques: modulation by atorvastatin. Stroke 37:2044–2053
Chen HN, Wang DJ, Ren MY et al (2012) TWEAK/Fn14 promotes the proliferation and collagen synthesis of rat cardiac fibroblasts via NF-кB pathway. Mol Biol Rep 39:8231–8241
Linzbach AJ (1960) Heart failure from the point of view of quantitative anatomy. Am J Cardiol 5:370–382
Kajstura J, Leri A, Finato N et al (1998) Myocyte proliferation in end-stage cardiac failure in humans. Proc Natl Acad Sci USA 95:8801–8805
Olivetti G, Melissari M, Balbi T et al (1994) Myocyte nuclear and possible cellular hyperplasia contribute to ventricular remodeling in the hypertrophic senescent heart in humans. J Am Coll Cardiol 24:140–149
Anversa P, Fitzpatrick D, Argani S et al (1991) Myocyte mitotic division in the aging mammalian rat heart. Circ Res 69:1159–1164
Capasso JM, Bruno S, Li P et al (1993) Myocyte DNA synthesis with aging: correlation with ventricular loading in rats. J Cell Physiol 155:635–648
Setoguchi M, Leri A, Wang S et al (1999) Activation of cyclins and cyclin-dependent kinases, DNA synthesis, and myocyte mitotic division in pacing-induced heart failure in dogs. Lab Invest 79:1545–1558
Sheikh F, Fandrich RR, Kardami E et al (1999) Overexpression of long or short FGFR-1 results in FGF-2-mediated proliferation in neonatal cardiac myocyte cultures. Cardiovasc Res 42:696–705
Bersell K, Arab S, Haring B et al (2009) Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell 138:257–270
Kehat I, Molkentin JD (2010) Molecular pathways underlying cardiac remodeling during pathophysiological stimulation. Circulation 122:2727–2735
Ritter O, Neyses L (2003) The molecular basis of myocardial hypertrophy and heart failure. Trends Mol Med 9:313–321
Swynghedauw B (1999) Molecular mechanisms of myocardial remodeling. Physiol Rev 79:215–262
Bover LC, Cardo-Vila M, Kuniyasu A et al (2007) A previously unrecognized protein–protein interaction between TWEAK and CD163: potential biological implications. J Immunol 178:8183–8194
Acknowledgments
This work was supported by grants from the Key Technologies R&D Program of Shandong Province (grant number 2007GG10002014) and the Natural Science Foundation of Shandong Province (Y2007C073).
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ren, MY., Sui, SJ. The role of TWEAK/Fn14 in cardiac remodeling. Mol Biol Rep 39, 9971–9977 (2012). https://doi.org/10.1007/s11033-012-1867-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-1867-6