Abstract
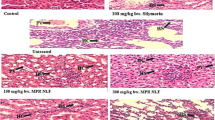
A wide number of pesticides, including highly persistent organochlorine compounds, such as lindane (γ-Hexachlorocyclohexane), have deteriorative effect on fauna and flora by inducing oxidative stress. Lindane induces cell damage by producing free radicals and reactive oxygen species. Quercetin, a dietary flavonoid, is ubiquitous in fruits and vegetables and plays an important role in human health by virtue of its antioxidant function. In this study the flavonoid quercetin was used to investigate its antioxidative effect against lindane induced oxidative stress in rats. The level of lipid peroxidation, reduced glutathione (GSH) were analysed in addition to the antioxidant enzymes such as catalase (CAT), glutathione peroxidase (GPx), superoxide dismutase (SOD) and glutathione-s-transferase (GST) activities in the liver and kidney tissue. Levels of hepatic marker enzymes in serum like Aspartate transaminase (AST), Alanine transaminase (ALT), Alkaline phosphatase (ALP) and Lactate dehydrogenase (LDH) and renal markers like serum creatinine and serum urea were estimated. Administration of Lindane induced histopathological alterations and increased levels of serum hepatic and renal markers and malondialdehyde (MDA) with a significant decrease in GSH content and CAT, SOD, GPx and GST activities. Cotreatment of quercetin along with lindane significantly decreased the lindane induced alteration in histology, serum hepatic and renal markers and MDA and also improved the cellular antioxidant status. The results show that Quercetin ameliorates Lindane induced oxidative stress in liver and kidney. The quercetin exhibited chemopreventive effect when administered along with lindane.









Similar content being viewed by others
Abbreviations
- ALP:
-
Alkaline phosphatase
- ALT:
-
Alanine transaminase
- ANOVA:
-
Analysis of variance
- AST:
-
Aspartate transaminase
- CAT:
-
Catalase
- CPCSEA:
-
Committee for the purpose of control and supervision of experiments and animals
- GPx:
-
Glutathione peroxidase
- GSH:
-
Reduced glutathione
- GST:
-
Glutathione-s-transferase
- H&E:
-
Hematoxylin and eosin
- LDH:
-
Lactate dehydrogenase
- LPO:
-
Lipid peroxidation
- MDA:
-
Malondialdehyde
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- TBARS:
-
Thio barbituric acid reactive substances
References
Donald DB, Block H, Wood J (1997) Role of ground water on hexachlorocyclohexane (lindane) detections in surface water in western Canada. Environ Toxicol Chem 16(9):1867–1872
Bintein S, Devillers J (1996) Evaluating the environmental fate of lindane in France. Chemosphere 32(12):2427–2440
Sang S, Petrovic S, Cuddeford V (1999) Lindane—a review of toxicity and environmental fate. World Wildl Fund Can 1–72
Piskac-Collier AL, Smith MA (2009) Lindane-induced generation of reactive oxygen species and depletion of glutathione do not result in necrosis in renal distal tubule cells. J Toxicol Environ Health Part A 72:1160–1167
Banerjee BD, Seth V, Bhattacharya A, Pasha ST, Chakraborty AK (1999) Biochemical effects of some pesticides on lipid peroxidation and free-radical scavengers. Toxicol Lett 107(1–3):33–47
Barros SB, Simizu K, Junqueira VB (1991) Liver lipid peroxidation-related parameters after short-term administration of hexachlorocyclohexane isomers to rats. Toxicol Lett 56(1–2):137–144
Bano M, Bhatt DK (2007) Neuroprotective role of a novel combination of certain antioxidants on lindane (g-HCH) induced toxicity in cerebrum in mice. Res J Agric Biol Sci 3(6):664–669
Andrews JE, Gray LE (1990) The effects of lindane and linuron on calcium metabolism, bone morphometry and the kidney in rats. Toxicology 60(1–2):99–107
Abdollahi M, Ranjbar A, Shadnia Sh, Nikfar Sh, Rezaie A (2004) Pesticides and oxidative stress: a review. Med Sci Monit 10:141–147
Daryani NE, Keramati MR, Daryani E (2008) Lindane-induced hepatotoxicity in human?: report of a rare case. Govaresh 13(1):58–59
Anilakumar KR, Saritha V, Khanum F, Bawa AS (2009) Ameliorative effect of ajwain extract on hexachlorocyclohexane induced lipid peroxidation in rat liver. Food Chem Toxicol 47:279–282
Husain SR, Cillard J, Cillard P (1987) Hydroxy radical scavenging activity of flavonoids. Phytochemistry 26(9):2489–2491
Osawa T, Katsuzaki H, Hagiwara Y, Hagiwara H, Shibamoto T (1992) A novel antioxidant isolated from young green barley leaves. J Agric Food Chem 40(7):1135–1138
Ohnishi M, Morishita H, Iwahashi H, Toda S, Shirataki Y, Kimura M, Kido R (1994) Inhibitory effects of chlorogenic acids on linoleic acid peroxidation and haemolysis. Phytochemistry 36(3):579–583
Duthie SJ, Collins AR, Duthie GG, Dobson VL (1997) Quercetin and myricetin protect against hydrogen peroxide-induced DNA damage (strand breaks and oxidised pyrimidines) in human lymphocytes. Mutat Res 393(3):223–231
Dok-Go H, Lee KH, Kim HJ, Lee EH, Lee J, Song JS, Lee YH, Jin C, Lee YS, Cho J (2003) Neuroprotective effects of antioxidative flavonoids, quercetin, (+)-dihydroquercetin and quercetin 3-methyl ether, isolated from Opuntia ficus-indica var. saboten. Brain Res 965(1–2):130–136
Ioku K, Tsushida T, Takei Y, Nakatani N, Terao J (1995) Antioxidative activity of quercetin and quercetin monoglucosides in solution and phospholipid bilayers. Biochim Biophys Acta 1234(1):99–104
Kefalas P, Kallithraka S, Parejo I, Makris DP (2003) Note: a comparative study on the in vitro antiradical activity and hydroxyl free radical scavenging activity in aged red wines. Food Sci Technol Int 9(6):383–387
Renugadevi J, MiltonPrabu S (2009) Ameliorative effect of quercetin against cadmium induced toxicity in liver of Wistar rats. J Cell Tissue Res 9:1665–1672
Morales AI, Vicente-Sanchez C, Santiago Sandoval JM, Egido J, Mayoral P, Arevalo M, Fernandez-Tagarro M, Lopez-Novoa JMF, Perez-Barriocanal F (2006) Protective effect of quercetin on experimental chronic cadmium nephrotoxicity in rats is based on its antioxidant properties. Food Chem Toxicol 44:2092–2100
Filipe P, Haigle J, Silva JN, Freitas J, Fernandes A, Mazière JC, Mazière C, Santus R, Morlière P (2004) Anti- and pro-oxidant effects of quercetin in copper-induced low density lipoprotein oxidation: quercetin as an effective antioxidant against pro-oxidant effects of urate. Eur J Biochem 271:1991–1999
Samanta L, Chainy GB (1997) Comparison of hexachlorocyclohexane-induced oxidative stress in the testis of immature and adult rats. Comp Biochem Physiol C: Pharmacol Toxicol Endocrinol 118:319–327
Sujatha R, Chitra KC, Latchoumycandane C, Mathur PP (2001) Effect of lindane on testicular antioxidant system and steroidogenic enzymes in adult rats. Asian J Androl 3:135–138
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Natelson S, Scott ML, Beffa C (1951) A rapid method for the estimation of urea in biological fluids. Am J Clin Pathol 21(3):275–281
Brod J, Sirota JH (1948) The renal clearance of endogenous creatinine in man. J Clin Invest 27(5):645–654
Bergmeyer HU, Bernt E, Mollering H, Pfleiderer G (1974) l-aspartate and l-asparagine. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol 4. Academic Press, Inc., New York, pp 1696–1700
Lowry OH, Robertson NR, Wu ML, Hixon WS, Crawfold EJ (1954) The quantivetative histochemistry of brain II enzyme measurements. J Biol Chem 207:19–37
King J (1965) In: Van D (ed) Practical clinical enzymology, pp 83–93
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47(3):469–474
Takahara S, Hamilton HB, Neel JV, Kobara TY, Ogura Y, Nishimura ET (1960) Hypocatalasemia: a new genetic carrier state. J Clin Invest 39:610–619
Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179(4073):588–590
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249(22):7130–7139
Moron MS, Depierre JW, Mannervik B (1979) Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta 582(1):67–78
Xavier R, Rekha K, Bairy KL (2004) Health perspective of pesticide exposure and dietary management. Malays J Nutr 10:39–51
Dorai T, Aggarwal BB (2004) Role of chemopreventive agents in cancer therapy. Cancer Lett 215:129–140
Ferraresi R, Troiano L, Roat E, Lugli E, Nemes E, Nasi M, Pinti M, Fernandez MI, Cooper EL, Cossarizza A (2005) Essential requirement of reduced glutathione (GSH) for the anti-oxidant effect of the flavonoid quercetin. Free Radic Res 39:1249–1258
Smith AG (1991) Chlorinated hydrocarbon insecticides. In: Hayes WJ Jr, Laws ER Jr (eds) Handbook of pesticide toxicology. Academic Press, Inc., New York, pp 3–6
Etim OE, Farombi EO, Usoh IF, Akpan EJ (2006) The protective effect of aloe vera juice on Lindane induced hepatotoxicity and Genotoxicity. Pak J Pharm Sci 19(4):333–337
Galisteo M, García-Saura MF, Jiménez R, Villar IC, Zarzuelo A, Vargas F, Duarte J (2004) Effects of chronic quercetin treatment on antioxidant defence system and oxidative status of deoxycorticosterone acetate-salt-hypertensive rats. Mol Cell Biochem 259:91–99
García-Saura MF, Galisteo M, Villar IC, Bermejo A, Zarzuelo A, Vargas F, Duarte J (2005) Effects of chronic quercetin treatment in experimental renovascular hypertension. Mol Chem Biochem 270:147–155
Suter P (1983) Three months toxicity study in rats with lindane. Research and consulting company AG, Itingen, Switzerland RCC project no. 005220
Renugadevi J, MiltonPrabu S (2010) Quercetin protects against oxidative stress-related renal dysfunction by cadmium in rats. Exp Toxicol Pathol 62:471–481
Rajesh MG, Latha MS (2004) Preliminary evaluations of the antihepatotoxic effect of Kamilari, a polyherbal formulation. J Ethnopharmacol 91:99–104
Videla LA, Troncoso P, Arisi ACM, Junqueira VBC (1997) Dose-dependent effects of acute lindane treatment on Kupffer cell function assessed in the isolated perfused rat liver. Xenobiotica 27:745–757
Perrone RD, Madias NE, Levey AS (1992) Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem 38(10):1933–1953
Newman DJ, Thakkar H, Edwards RG, Wilkie M, White T, Grubb AO, Price CP (1995) Serum cystatin C measured by automated immunoassay: a more sensitive marker of changes in GFR than serum creatinine. Kidney Int 47(1):312–318
Katz D, Mazor D, Dvilansky A, Meyerstein N (1996) Effect of radiation on red cell membrane and intracellular oxidative defense systems. Free Radic Res 24(3):199–204
Enginar H, Avci G, Eryavuz A, Kaya E, Kucukkurt I, Fidan AF (2006) Effect of Yucca schidigera extract on lipid peroxidation and antioxidant activity in rabbits exposed to γ-radiation. Revue Med Vet 157:415–417
Sahoo A, Samanta L, Chainy GBN (2000) Mediation of oxidative stress in HCH-induced neurotoxicity in rat. Arch Environ Contam Toxicol 39:7–12
Moran JF, Klucas RV, Grayer RJ, Abian J, Becana M (1997) Complexes of iron with phenolic compounds from soybean nodules and other legume tissues: prooxidant and antioxidant properties. Free Radic Biol Med 22(5):861–870
Bischoff SC (2008) Quercetin: potentials in the prevention and therapy of disease. Curr Opin Clin Nutr Metab Care 11:733–740
Coskun O, Kanter M, Ahmetkorkmaz A, Sukruoter S (2005) Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and β-cell damage in rat pancreas. Pharmacol Res 51:117–123
Fidan AF, Cigeri IH, Baysu-Sozbilir N, Kucukkurt I, Yuksel H, Keles H (2008) The effect of the dose-dependent γ-Hexachlorocyclohexane (lindane) on blood and tissue antioxidant defence systems, lipid peroxidation and histopathological changes in rats. J Anim Vet Adv 7:1480–1488
Anilakumar KR, Khanum F (2009) Effect of bee wax polyphenols on hexocyclohexane-induced oxidative stress in rat liver. Int J Integ Biol 7(3):156–159
Johri A, Dhawan A, Singh RL, Parmar D (2008) Persistence in alterations in the ontogeny of cerebral and hepatic cytochrome P450s following prenatal exposure to low doses of lindane. Toxicol Sci 101:331–340
Kaneno T, Baba N (1999) Protective effect of flavonoids on endothelial cells against linoleic acid hydroperoxide-induced toxicity. Biosci Biotechnol Biochem 63(2):323–328
Chatuphonprasert W, Kondo S, Jarukamjorn K, Kawasaki Y, Sakuma T, Nemoto N (2010) Potent modification of inducible CYP1A1 expression by flavonoids. Biol Pharm Bull 33(10):1698–1703
Boots AW, Kubben N, Haenen GR, Bast A (2003) Oxidized quercetin reacts with thiols rather than with ascorbate: implication for quercetin supplementation. Biochem Biophys Res Commun 308:560–565
Saija A, Scalese M, Lanza M, Marzullo D, Bonina F, Castelli F (1995) Flavonoids as antioxidant agents: Importance of their interaction with biomembranes. Free Radic Biol Med 19:481–486
Moridani MY, Pourahmad J, Bui H, Siraki A, O’Brien PJ (2003) Dietary flavonoid iron complexes as cytoprotective superoxide radical scavengers. Free Radic Biol Med 34:243–253
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Padma, V.V., Baskaran, R., Roopesh, R.S. et al. Quercetin attenuates lindane induced oxidative stress in wistar rats. Mol Biol Rep 39, 6895–6905 (2012). https://doi.org/10.1007/s11033-012-1516-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-1516-0