Abstract
Liver and kidney diseases are becoming order of the day in both developed and developing countries as a result of environmental pollutants such as lead. Restorative activities of methanol and methanol/acetone phenolic-rich extracts (MPR and MAPR, respectively) of the N. latifolia fruit (NLF) on lead acetate-induced liver and kidney damaged were assessed in Wistar rats. The antioxidant activities of both phenolic-rich extracts of NLF were also carried out using standard methods. Seven groups of Wistar rats comprising of 5 rats each were used for the study and 1000 mg/kg body weight (bw.) of lead acetate solution was administered orally to the 6 groups of animals to induce liver and kidney damage. The high and low dosages of 300 and of 150 mg/kg body weight (bw.) of both MPR and MAPR were administered orally to four groups for 14 days along positive (100 mg/kg bw. of silymarin), negative (treated with the placebo) and naïve control (non-induced). The percentage DPPH radical scavenging activities, ferric reducing antioxidant power and percentage inhibition of lipid peroxidation show high antioxidants activities dose-dependently. Furthermore, administration of lead acetate significantly (p > 0.05) reduces the weight gain and elevates the liver and kidney relative weight as well as their respective damage biomarkers with distortions in their histologies. However, treatment with MPR and MAPR resulted in significant (p < 0.05) improve in the percentage body weight gain, relative liver and kidney weight as well as restoration of the activities of the liver and kidney functions biomarkers of the treated animals. Likewise, lesser hepatic and renal cells injury were also observed in the treated groups with MAPR being more active at high dosage which significantly (p < 0.05) compared well with normal group. Hence, the phenolics content of the N. latifolia fruit can be exploited further for drug development for the management kidney and liver damage arise from lead-induced toxicity.
Similar content being viewed by others
Introductions
Environmental pollution is one of the factors that cause serious health problems on living organisms [1]. At present, lead is among the heavy metals that contributes to the leading causes of environmental pollution [1, 2]. Hence, it has been rated among ten chemicals of major public health concern by the World Health Organization [3]. Lead has been confirmed to generate an extensive range of adverse physiological and biochemical effects which involve several cells, tissues, organs and systems malfunctioning in all mammals [1]. Many studies have implicated lead in stimulating overproduction of reactive oxygen species (ROS) which result to tissues degeneration that can lead to a substantial health challenge [4].
Liver and kidney are very important and delicate organs due to their metabolic and excretory roles towards several poisonous substances [5]. Therefore, several form of liver and kidney ailments that lead to mortality keep increasing globally. According to the World Health Organization (WHO) global health estimate of 2015, chronic liver disease ranks as the 16th main cause of illness globally [6, 7]. In 2017, the figure of mortality linked with chronic kidney diseases (CKD) or CKD-related complications was projected to be 1.2 million and this account for 4.6% of world-wide deaths [8]. Most of the chelating agents such as disodium edetate, dimercaprol, succimer among others used to curb lead induced toxicity exhibit various setbacks. These hinderances include toxicity, inability to chelate the accumulated lead in some tissues, prooxidant properties of some of the chelating agents as well as their high cost. For these reasons, it is justifiable to search for alternative drugs from natural sources.
Phenolic compounds are secondary metabolites that can be sourced from plant and they are one of the major sources of antioxidant which can protect the cells from oxidative impairment triggered by free-radicals [9]. Phenolics are known to serve as reducing agents, metal chelators, singlet oxygen quenchers, radical scavengers and hydrogen donors [10]. However, in order to extract maximum quantity of these secondary metabolites from several plants, selected solvents of different polarities must be used coupled with suitable method of phytoconstituent extraction [11].
Nauclea latifolia (family Rubiaceae) is commonly called African peach (English), Ubulu inu (Igbo), Tafashiya, Marga, tabashiya or tuwon biri (Hausa), Egbesi (Yoruba), Mbom-ibong (Ibibio) in Nigeria West Africa [12]. It is a small tree or compact shrub from tropical Asia and Africa. It is a scattering, perennial, multi-stemmed shrubs. It possesses large red ball fruit with long projecting stamens and flower. It projected to an altitude of 200 m and over 20 ft. high. The plant has rough bark and leaves that measure 7 by 4–5 in [13].. The fruits are usually plump, shallow-pitched, embedded with many seeds surrounded by a pink edible, sweet- sour pulp. The seeds are usually small, ovoid, numerous and brownish with a pleasant taste but could be emetic if taken in excess [14]. Folklorically, Africans are using various parts of the plant as a remedy for pain, dental caries, diarrhoea, diabetes and septic mouth [15]. It is also used for the treatment of debility, malaria, gastrointestinal disorders, hypertension, sleeping sickness, leprosy and prolonged menstrual flow [16, 17] and the sticks are used as chewing stick for tuberculosis remedy [18]. Pharmacologically, N. latifolia have been reported to have antimicrobial [19], antiulcer [20], antihypertensive [21], hypocholesterolaemia [22], anti-diabetes [23, 24], hepatoprotective [25], wound healings ([26].), Anti-abortifacient [27], antioxidant [28], antimalarial [24], antinflamation and antidiabetes [29] activities among others.
Our previous study showed that phenolic-rich extracts of N. latifolia fruit ameliorates lead acetate-induced haematology and lung tissues toxicity in male Wistar rats [11]. Therefore, it is not out of place to evaluate the restorative effects of phenolics rich-extracts from N. latifolia fruit on lead acetate-induced liver and kidney damage in Wistar rats via changes in liver damaged markers, kidney damaged markers as well as the histology of both organs.
Materials and methods
Materials
Plant collection
Fresh, matured fruits of N. latifolia were collected from Bosso Estate in Bosso Local Government area (Niger State, Nigeria) on Latitude: 9.6550611 and Longitude:6.5138343. The fruit was identified by a Senior Botanist of the Biological Science Department of Federal University of Technology Minna with voucher number: FUT.MIN/SLS/PB-019-016 and the specimen was deposited at the herbarium of the University.
Kits, chemicals and reagents
The biochemical kits used were obtained from AGAPE Laboratories, Hombrechtikon, Switzerland. The chemicals and reagents used were obtained from Sigma-Aldrich Chemical Company St. Louis, USA. The reagents include methanol, acetone, ethylacetate, Ferric chloride, DPPH, lead acetate, hematoxylin and eosin among others.
Experimental animals
Male Wistar rats (125.00 ± 3.16) g were obtained from the animal facility of Federal University of Technology Minna (FUT.MINNA), Niger State, Nigeria. The animals were housed in polypropylene cages under a controlled environment with 12 h light/dark cycles, temperature of 28 ± 3 °C and relative humidity of 45–50%. The animals were fed on pelleted diet (Vital Feeds, Jos Nigeria) with supply of water ad libitum. The experiment was conducted according to protocol review (1997) of Canadian Council on Animal Care and use guidelines. Ethical clearance number 0000012EAU was given by FUT.MINNA/Nigeria Ethical Review Committee.
Methods
Sample preparations
Matured Fruits of N. latifolia were washed, sliced and dried at room temperature (28 ± 2) °C. The dried fruits were pulverised using electric blender (Binatone BLG 450, United Kingdom). The milled sample was kept in an air tight plastic vessel at room temperature (28 ± 2) °C until further use.
Phenolics extraction from the N. latifolia fruits
Fifty grams (50 g) of the powdered sample was positioned in a round bottom flask and steeped with 400 mL of 70% methanol and another 50 g of the fruits sample was steeped with 40% acetone and 60% methanol mixture at temperature of (28 ± 2) °C for 72 h with occasional mixing. The extracts were filtered with Whatman filter paper after which the methanol or methanol/acetone solvent were evaporated at low pressure in a rotary vacuum evaporator. The filtrate of N. latifolia fruit gotten were freeze-dried and phenolics rich extract obtained was refrigerated at − 4 °C.
In vitro antioxidant activities of phenolics from N. latifolia fruits
The method of Oyaizu [30] was used for the determinations of scavenging activity of 1, 1-diphenyl-2-picryl hydrazyl (DPPH) and the ferric reducing antioxidant power of the phenolics from N. latifolia fruits while Halliwell et al. [31] method was used to determine percentage inhibition of lipid peroxidation of the extract using a modified thiobarbituric acid reactive substances (TBARS) assay as all reported by Ibrahim et al. [32].
Animal grouping for lead acetate-induced hepato-renal toxicity study
The Wister rats were grouped into seven of five rats each as shown below;
Group A (Naïve Control): 0.5 mL/kg bw. of Normal saline.
Group B (Positive Control): Lead acetate (1000 mg/kg bw.) + 100 mg/kg bw. Silymarin.
Group C (Negative Control): Lead acetate (1000 mg/kg bw.) + 0.5 mL/kg bw. of Normal Saline.
Group D (150 mg/kg bw. of MPR): Lead acetate (1000 mg/kg bw.) + 150 mg/kg bw. Methanol extract of phenolic-rich N. latifolia fruit.
Group E (300 mg/kg bw. of MPR): Lead acetate (1000 mg/kg bw.) + 300 mg/kg bw. Methanol extract of phenolic-rich N. latifolia fruit.
Group F (150 mg/kg bw. of MAPR): Lead acetate (1000 mg/kg bw.) + 150 mg/kg bw. Methanol/Acetone extract of phenolic-rich N. latifolia fruit.
Group G (300 mg/kg bw. of MAPR): Lead acetate (1000 mg/kg bw.) + 300 mg/kg bw. Methanol extract of phenolic-rich N. latifolia fruit.
The 1000 mg/kg bw. of lead acetate was orally administered to induce lead toxicity in six groups for 14 days after weighing the animals. Each extract of 150 mg/kg bw. and 300 mg/kg bw. were administered to four of the groups after one hour of lead acetate administration. One of the remaining two groups of the lead induced toxic group was treated with 100 mg/kg bw. of silymarin (Standard drug) while the other one (Untreated) was given 2 mL/kg bw. of 0.9% saline solution. The animals were weighed and euthanized by cardiac puncture at 15th day after an overnight fasting while blood, liver and kidney were collected for further analysis.
Serum, liver and kidney preparations
Serum was prepared by using the procedure described by Yakubu et al. [33]. Briefly, the rats were euthanized by cervical dislocation at day 15th and the blood samples were collected by cardiac puncture from the inferior vena cava of the heart into serum sample bottles. The blood samples were allowed to clot at room temperature and serum was obtained by centrifuging at 3000 rpm for 10 min. The liver and kidney were collected, washed blotted and weighed as described by Busari et al. [34].
Biochemicals assays
Marker enzymes and metabolites of liver and kidney damage
All enzymes and metabolites of liver and kidney damage were determined by using appropriate biochemical kits (Randox and Agape commercial kits) according to the following methods; sodium [35], Potassium [36] and Chloride [37], alkaline phosphatase [38], alanine amino transferase and aspartate amino [39], total protein [40], albumin [41], urea [42], creatinine [36],
Histologies of the liver and kidney
A section of liver and kidney tissues was quickly fixed in 10% neutral buffered formalin solution prior to at least 24 h of examination. The fixed specimen was processed by using conventional paraffin embedding technique (dehydration via ascending grades of C2H5OH, clearing using chloroform and embedding with paraffin wax at 60 °C. Paraffin blocks was prepared from which 3–4 μm thick sections was gotten and stained with haematoxylin and eosin (H&E). The histology observations were then made under light microscope at Magnification × 40.
Data analysis
The analysis of variance (ANOVA) followed by Post hoc Duncan multiple comparisons test (Statistical Package for Social Sciences, version 22.0, SPSS Inc., Chicago, IL, USA) at 95% confidence interval was used on the data obtained with p-value less than 0.05 was considered significant difference. The data were expressed as mean ± standard error mean of five replicates.
Results
Percentage of scavenging activity of methanol and methanol/acetone phenolic-rich extracts (MPR and MAPR) of NLF against 1, 1-Diphenyl-2-Picryl Hydrazyl radical (DPPH)
The % DPPH Scavenging activities of methanol and methanol/acetone phenolic-rich extracts of the NLF are shown in Table 1. Both extracts and the standard (vitamin C) exhibit dose dependent % DPPH Scavenging activities. The % DPPH Scavenging activities are in following order; methanol extract < methanol/acetone extract < vitamin C with the highest at 500 μg/mL (42.32 < 62.32 < 91.35).
Values are Means of five replicates ± standard error mean. Values with different superscripts and on the same column are significantly different (p < 0.05).
Ferric reducing antioxidant power (FRAP) of methanol and methanol/acetone phenolic-rich extracts of NLF
The ferric reducing antioxidant power of methanol and methanol/acetone phenolic-rich extracts of the NLF are shown in Table 2. Both extracts and the standard (vitamin C) also exhibit dose dependent FRAP as it was observed in % DPPH Scavenging activities. The FRAP of methanol and methanol/acetone phenolic-rich extracts of NLF are in following order; methanol extract < methanol/acetone extract < vitamin C with significant high value of FRAP at 500 μg/mL (0.82 < 0.88 < 2.50).
Values are Means of five replicates ± standard error mean. Values with different superscripts and on the same column are significantly different (p < 0.05).
Inhibition of lipid peroxidation of methanol and methanol/acetone phenolic-rich extracts of NLF
The Inhibition of lipid peroxidation of methanol and methanol/acetone phenolic-rich extracts of the NLF are shown in Table 3. No significant differences (p > 0.05) between the Inhibition of lipid peroxidation of methanol, methanol/acetone phenolic-rich extracts of the NLF and the standard (vitamin C) at 125 μg/mL. However, Inhibition of lipid peroxidation of methanol/acetone phenolic-rich extracts of the NLF is significant higher (p < 0.05) when compared with both methanol and vitamin C at 500 μg/mL respectively (47.35 > 42.88 > 41.73).
methanol/acetone phenolic-rich extract of the NLF is significant higher (p < 0.05) when compared with both methanol and vitamin C at 500 μg/mL respectively (47.35 > 42.88 > 41.73).
Values are Means of five replicates ± standard error mean. Values with different superscripts and on the same column are significantly different (p < 0.05).
Changes in body weight, relative liver and kidney weight of male Wistar rats administered with lead acetate and treated with methanol and methanol/acetone phenolic-rich extracts of N. latifolia fruit
The body weight changes along with relative weights of liver and kidney were shown in Tables 4 and 5 respectively. Oral administration of lead acetate to the experimental animals significantly decreases (p > 0.05) the body weight with significant increase in relative weight of liver and kidney of rats in negative control group when compared with other treated groups. In most cases animals treated with 300 mg/kg bw. MAPR NLF are significantly (p < 0.05) compared well with those in normal groups.
Values are Means of five replicates ± standard error mean. Values with different superscripts and on the same column are significantly different (p < 0.05).
Values are Means of five replicates ± standard error mean. Values with different superscripts and on the same column are significantly different (p < 0.05).
Liver and kidney damage biomarkers of male Wistar rats administered with lead acetate and treated with methanol and methanol/acetone phenolic-rich extracts of N. latifolia fruit
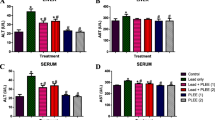
The biomarkers for liver damage in Wistar rats administered with lead acetate and treated with methanol and methanol/acetone phenolic-rich extracts of N. latifolia fruit is presented in Table 6. Administration of lead acetate significantly increase (p > 0.05) the ALP (47.50 U/L), AST (22.70 U/L), and ALT (27.50 U/L) enzymes as well as conjugated bilirubin (4.45 mg/dL) while total protein (1.45 g/dL) and albumin (g/dL) reduce significantly when compared with control group. Treatment with lead acetate and treated with methanol and methanol/acetone phenolic-rich extracts of N. latifolia fruit however significantly (p > 0.05) restore the level of these aforementioned parameters at all dosages. Moreover, methanol/acetone phenolic-rich extracts of N. latifolia fruit at dosage 300 mg/kg bw. performed excellently well when compare to methanol phenolic-rich extracts and the standard drug (silymarin) at chosen dosages.
The biomarkers for kidney damage in Wistar rats administered with lead acetate and treated with methanol and methanol/acetone phenolic-rich extracts of N. latifolia fruit is presented in Table 7. Administration of lead acetate significantly increase (p > 0.05) the urea (98 mg/dL), sodium (136.50 mmol/L), potassium (15.10 mmol/L), chloride (99.50 mmol/L) enzymes as well as creatinine (4.00 mg/dL) when compared with control group. Treatment with lead acetate and treated with methanol and methanol/acetone phenolic-rich extracts of N. latifolia fruit however significantly (p > 0.05) restored the level of these kidney damage biomarkers at all dosages. Methanol/acetone phenolic-rich extracts of N. latifolia fruit at dosage 300 mg/kg bw. performed excellently well when compared with methanol phenolic-rich extracts and the standard drug (silymarin) at chosen dosages but not comparable to un-induced control group.
Values are Means of five replicates ± standard error mean. Values with different superscripts and on the same column are significantly different (p < 0.05).
Values are Means of five replicates ± standard error mean. Values with different superscripts and on the same column are significantly different (p < 0.05).
Histology of the liver and kidney of male Wistar rats administered with lead acetate and treated with methanol and methanol/ acetone phenolic-rich extract of N. latifolia fruits
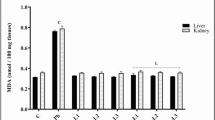
The administration of lead acetate caused marked hepatocytes necrosis, central vein congestion, vacuolar degeneration and scattered lymphocytes in between the hepatocytes and in the sinusoids. and in the histology of the liver tissue of the lead acetate treated group (Figure 1) while their kidney tissue showed capsular and tubular degeneration (Figure 2). However, the methanol and methanol/ acetone phenolic-rich extract of N. latifolia fruits treated groups showed mild hepatocytes necrosis, mild central vein congestion, mild vacuolar degeneration and hepatocytes regeneration which is comparable to the reference drug (100 mg/kg bw silymarin) treated group.
Discussions
Lead toxicity has proven to be one of the leading global problems associated with a number of pathological disorders which include neurotoxicity, inflammation, liver and kidneys damages, immunomodulation, hypertension, cardiovascular diseases, and anaemia [43]. Owing to the pharmacological properties alongside with little or no toxicity attributed to the compounds of natural origins possessing antioxidant activities, the search for such compounds is warranted [44].
A number of studies are available backing the implication of oxidative stress in several human and animal diseases. In human or animal body, free radicals are produced naturally from the metabolism of nutrients and indigenous compounds. These free radicals can be eliminated or quenched by antioxidants which can either be natural or synthetic [32]. According to Ibrahim et al. [32], when oxidative stress set in, free radicals cause oxidation of vital biomolecules such as lipids, proteins, carbohydrates and deoxyribonucleic acid (DNA) in healthy human cells leading to diverse physiological disorders.
Growth has been considered a very important indicator of living organisms health status. Lead as a toxic metal has been reported to alter body weight and relative organ weight of animals as the ingestion of this toxic substances has been previously linked with poor growth rate [34]. In addition, ingestion of lead was reported to have lead to increased relative organ weights as a result of necrosis and apoptosis triggered by the accumulation of lipids in such organs [45, 46]. Likewise, relative organs weight is one of the markers to indicate oedema, atrophy, or hypertrophy of some organs [34]. Hence, the reversion of body weight and relative organs weights of the experimental animals treated with phenolic extract when compared with negative control group in this study infers that the extracts were able to prevent hepatomegaly and kidney enlargement caused by lead-induced toxicity. The higher protective activities of the extracts at dose of 300 mg/kg bw could be due to increased concentrations of phenolic compounds at this dosage which in turn maximumly prevent, protect or repair the tissues from lead induced oxidative damage.
Natural antioxidant compounds such as phenolics possess free radicals-quenching power which in turn results in the prevention of organs damage emanating from the actions of free radicals. The antioxidant activities of the phenolic compounds have been shown to be through a number of mechanisms which are structure-dependent, and these include: inhibition of the activities of the enzymes involved in the production of reactive oxygen species, stabilizing produced free radicals or chelating metal ions, or up-regulation of genes involved in the production of antioxidant enzymes [47]. The ability of the extracts (methanol and methanol/acetone phenolic-rich extracts) to exhibit excellent antioxidant properties through percentage DPPH scavenging activities, ferric reducing antioxidant power and inhibition of lipids peroxidation might be as a result of their phenolics content. As such, higher antioxidant activity of methanol/acetone phenolic-rich extract could be due to its higher phenolics content. This result agrees with the report of Busari et al. [11] who reported higher phenolics content (phenols, flavonoids and tannins) of methanol/acetone extract of NLF than its methanol extract. The higher activities (in vivo) of MAPR actually corresponds to its antioxidant activities (in vitro) which could be through stabilization of free radicals or metal chelation capacity (by donating hydrogen atom or electron to the free radical) which depend on the quantity of phenolic compounds present in the extract.
Liver is considered the major target for numerous toxicants such as lead. Environmental and occupational exposure to lead can result in many changes in the architecture of the liver [48]. Autopsy reports of humans exposed to lead indicated that liver is the principal reservoir (33%) of lead, followed by kidneys. According to Adaramoye et al. [49], biochemical parameters such as total protein, albumin, bilirubin, AST, ALT, and ALP are major biomarkers for liver functions and integrity. In acute hepatotoxicity or mild hepato-cellular damage, there is upsurge levels of these biochemical markers, but are observed to decrease with long-time intoxication due to liver damage [34]. In this study, the oral administration of lead acetate resulted in significant increased levels ALP, AST, ALT and conjugated bilirubin, while significant decrease in the levels of total protein and albumins was concomitantly observed. Liver structural integrity damage has been associated with increased levels of AST and ALT resulting from toxicants such as lead. The function of ALP in maintaining integrity of plasma membrane for proper functioning of organs [50]. In our study, a decrease in total protein level was observed in the liver tissues, which agrees with the report of El-Zayat et al. [51], who observed decrease in hepatic total protein levels resulting from lead intoxication. Inhibition of protein synthesis caused by lead may be as a result of its ability to cause damage or mutation to DNA or RNA [52]. Lead causes DNA mutation via base pair mutation, oxygen radical attack on DNA or deletion. In addition, Pb2+ disturbs homeostasis of Ca2+ intracellularly, and causes damage of endoplasmic reticulum resulting in reduced protein synthesis. Reduced albumin level was observed in rats treated with Pb owing to the decreased hepatic protein synthesis. The hypoalbuminemia observed in this study is in agreement with the study of Lakshmi et al. [53]. Offor et al. [54], bilirubin has been implicated in the protection of cell membrane against oxidative damage induced by metals. Thus, increased bilirubin levels observed in rats treated with lead acetate may be due to excessive destruction of heme and blockage of biliary tract resulting in the inhibition of the conjugation reaction and the release unconjugated bilirubin from damaged hepatocytes [50].
However, oral administration of methanol and methanol/acetone phenolic-rich extracts of NLF ameliorated the liver damages by reversing abnormal increase or decrease in the levels of the liver biomarkers. This effect also reflected in the liver histology of the rats treated with the extracts. The rats treated with the extracts showed mild hepatocytes necrosis, central vein congestion, vacuolar degeneration as well a gradual hepatocytes regeneration. The excellent and most promising activity observed with methanol/acetone phenolic-rich extract of NLF was not surprising due to its excellent exhibition of in vitro antioxidant activity when compared to the methanol phenolic-rich extract of NLF. Phenolic compounds such as tannins, flavonoids and other phenolics possess ability to quench free radicals such reactive oxygen species (ROS), which in turn prevent oxidation of essential biomolecules [32]. The chelating properties attributed to phenolics could be a contributing factor to the prevention of DNA and RNA damage, which would have been inflicted by lead via its interaction with intracellular Ca2+ signalling and the destruction of hepatic endoplasmic reticulum.
The increased levels of blood urea and creatinine in lead acetate-treated rats implies inability of the kidneys to excrete these by-products leading to their elevated levels in the blood and decreased excretion in urine [55]. Long-time exposure to lead resulted in the retention of electrolytes evidenced by upsurge levels of sodium, potassium and chloride since lead has been implicated in distortion of renal tubular transport mechanisms. The other possible way that might result in elevated levels of these electrolytes is the degeneration of functioning nephrons that stimulate a number of adaptive processes in the augmented rates of electrolytes reabsorption [56, 57]. Nonetheless, treatment with the phenolic-rich extracts of NLF restored all the abnormalities in the levels of these biomarkers with methanol/acetone phenolic-rich extracts having higher activity and brought the levels of these parameters close to normal and can be well compared with standard drug, silymarin. The histology of the kidneys validates the reversion of the kidneys damage via manifestation of less capsular and tubular degeneration alongside decreased inflammatory cell infiltration. The ability of the phenolic-rich extracts to play roles of reducing agents, metal chelators, singlet oxygen quenchers, radical scavengers and hydrogen donors could be a collective factor that contributed to the reversal of liver and kidneys damage resulting from lead-induced toxicity, by normalizing the levels of their biomarkers.
Apart from the previously mentioned mechanisms through which phenolic compounds could elicit their actions; Barrera et al. [58] also suggests that phenolic compounds can also exhibit hepatoprotective effects by upregulating Nrf2 signalling, a known transcription factor that activates the transcription of genes involved in cytoprotection [59, 60]. These antioxidant compounds were also observed to simultaneously downregulated NF-kB [61], a type of transcription factor that activates the transcription of inflammation-related genes [62]. Thus, hepato-renal protective effects of the phenolic-rich extracts which resulted into amelioration of lead-induced hepato-renal injury in extracts treated rats in this research could be as a result of upregulation of Nrf2 and/or downregulation of NF-kB transcription factors as suggested by aforementioned researchers.
Conclusions
It can be concluded that phenolic-rich extracts from Nauclea latifolia fruit exhibit ameliorative effects on lead acetate-induced liver and kidney toxicity in male Wistar rats with the exhibition of higher activities in methanol/acetone phenolic-rich extracts of the NLF.
References
Mancuso F, Arato I, Lilli C, Bellucci C, Bodo M, Calvitti M, et al. Acute effects of lead on porcine neonatal Sertoli cells in vitro. Toxicol in Vitro. 2018;48:45–52. https://doi.org/10.1016/j.tiv.2017.12.013.
Reche C, Moreno T, Amato F, Viana M, Van Drooge BL, Chuang HC, et al. A multidisciplinary approach to characterise exposure risk and toxicological effects of PM10 and PM2. 5 samples in urban environments. Ecotoxicol Environ Saf. 2012;78:327–35. https://doi.org/10.1016/j.ecoenv.2011.11.043.
World Health Organization. Ten chemicals of public health concern. World Health Organization Fact Sheets. 2020.
Mustafa SA, Al-Rudainy AJ, Al-Samawi SM. Histopathology and level of bioaccumulation of some heavy metals In Fish, Carasobarbus luteus and Cyprinus carpio tissues Caught from Tigris River, Baghdad. Iraqi J Agric Sci. 2020;51(2):698–704.
Ahmed MB, Islam SU, Lee YS. Concomitant Drug Treatment and Elimination in the RCC-affected Kidneys: Can We Kill Two Birds with One Stone?. Curr Drug Metab. 2020;21(13):1009–21.
World Health Organization. Global Health Estimates 2015: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2015. Geneva: World Health Organization; 2016.
Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70(1):151–71. https://doi.org/10.1016/j.jhep.2018.09.014.
Bikbov B, Purcell CA, Levey AS, Smith M, Abdoli A, Abebe M, et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2020;395(10225):709–33. https://doi.org/10.1016/S0140-6736(20)30045-3.
Hamzah RU, Jigam AA, Makun HA, Egwim EC, Kabiru AY, Madaki FM. Secondary metabolites and in-vitro antioxidant properties of methanol extracts of fruits of Annona senegalensis, Curcubita pepo L, Cucumi Melo inodorous and Sarcocephalus latifolius. Nigerian Journal of Technological Research. 2018;13(1):89–96. https://doi.org/10.4314/njtr.v13i1.9.
Wong PY, Kitts DD. Studies on the dual antioxidant and antibacterial properties of parsley (Petroselinum crispum) and cilantro (Coriandrum sativum) extracts. Food Chem. 2006;97(3):505–15. https://doi.org/10.1016/j.foodchem.2005.05.031.
Busari MB, Hamzah RU, Muhammad HL, Yusuf RS, Adeniyi JO, Ibrahim YO, et al. Phenolics-rich extracts of Nauclea latifolia fruit ameliorates lead acetate-induced haematology and lung tissues toxicity in male Wistar rats. Scientific African. 2021;11:e00686.
Arise RO, Akintola AA, Olarinoye JB, Balogun EA. Effects of aqueous extract of Nauclea latifolia stem on lipid profile and some enzymes of rat liver and kidney. Int J Pharmacol. 2012;8(5):389–95. https://doi.org/10.3923/ijp.2012.389.395.
Balogun ME, Besong EE, Obu DC, Obu MSU, Djobissie SFA. Nauclea latifolia: A Medicinal, Economic and Pharmacological Review. International Journal of Plant Research. 2016;6(2):34–52.
Iwu MM. African medicinal plants (pp. 109–110). Maryland: CRC Press; 1993.
Gidado A, Ameh DA, Atawodi SE. Effect of Nauclea latifolia leaves aqueous extracts on blood glucose levels of normal and alloxan-induced diabetic rats. Afr J Biotechnol. 2005;4(1):91–3.
Elujova A. Female infertility in the hands of traditional birch attendants in South-Western Nigeria. Fitoterapia (Milano). 1995;66(3):239–48.
Kerharo J. The beliefs and traditional practices in the treatment of sleeping sickness in West Africa. Bulletin de la Societe medicale d'Afrique noire de langue francaise. 1974;19(4):400–10.
Nwaehujor CO, Onyenweaku EO, Ezeigbo II, Asuzu OV. Evaluation of the hepato-protective potential of Sarcocephalus latifolius leaf methanol extracts in paracetamol-induced liver damage of mice. Comp Clin Pathol. 2016;25(5):1053–9. https://doi.org/10.1007/s00580-016-2310-5.
Nworgu ZAM, Onwukaeme DN, Afolayan AJ, Ameachina FC, Ayinde BA. Preliminary studies of blood pressure lowering effect of Nauclea latifolia in rats. Afr J Pharm Pharmacol. 2008;2(2):037–41.
Odey MO, Johnson JT, Iwara IA, Gauje B, Akpan NS, Luke UO, et al. Effect of antihypertensive treatment with root and stem bark extracts of Nauclea latifolia on serum lipid profile. GJP and A Sc and Tech. 2012;214:78–84.
Omale J, Haruna HU. Hypocholesterolemic effects of Nauclea latifolia(Smith) fruit studied in albino rats. Am J Trop Med. 2011;1(1):11–21.
Kadiri H, Adegor E, Asagba S. Effect of aqueous Nauclea pobeguinii leaf extract on rats induced with hepatic injury. Res J Med Plant. 2007;1(4):139–43. https://doi.org/10.3923/rjmp.2007.139.143.
Antia BS, Okokon JE. Phytochemical composition and antidiabetic activity of ethanol root extract of Nauclea latifolia. J Phytopharmacol. 2014;3:52–6.
Effiong GS, Akpan HD. The effect of Nauclea latifolia leaf extract on some biochemical parameters in streptozotocin diabetic rat models. J Med Med Sci. 2015;6(3):47–52.
Udeh SC, Madubunyi II. Hepatoprotective activities of methanolic extract of Nauclea latifolia. Agro-Science. 2008;7(1):72–7. https://doi.org/10.4314/as.v7i1.1588.
Udobre AS, Usifoh CO, Eseyin OA, Udoh AE, Awofisayo OA, Akpan AE. The wound healing activity of methanol extract of the stem bark of Nauclea latifolia. Int J Pharm Bio Sci. 2012;3(3):136–9.
Nworgu ZAM, Owolabi OJ, Atomah JE. Effect of the ethanolic extract of Nauclea latifolia (family: Rubiaceae) on the isolated uterus of non-pregnant rats. International Journal of Green Pharmacy. 2010;4(1):48. https://doi.org/10.4103/0973-8258.62162.
Mordi JC, Ojieh AE, Uzuegbu UE, Onyesom I, Onokurafe F. Changes in ocular oxidative indices in plasmodium berghei infected mice treated with aqueous leaf extract of Nauclea latifolia. Bioscience Biotechnology Research Communications. 2014;7(1):1–6.
Iheagwam FN, Israel EN, Kayode KO, DeCampos OC, Ogunlana OO, Chinedu SN. Nauclea latifolia Sm. Leaf extracts extenuates free radicals, inflammation, and diabetes-linked enzymes. Oxidative Med Cell Longev. 2020, 2020;
Oyaizu M. Studies on products of browning reaction antioxidative activities of products of browning reaction prepared from glucosamine. The Japanese journal of nutrition and dietetics. 1986;44(6):307–15. https://doi.org/10.5264/eiyogakuzashi.44.307.
Halliwell B, Chirico S. Lipid peroxidation: its mechanism, measurement, and significance. Am J Clin Nutr. 1993;57(5):715S–25S.
Ibrahim Y, Busari M, Yusuf R, Hamzah R. In vitro antioxidant activities of ethanol, ethyl acetate and n-hexane extracts of Mangifera indica leaves. Tanzania Journal of Science. 2020;46(3):628–35.
Yakubu MT, Oladiji AT, Akanji MA. Mode of cellular toxicity of aqueous extract of Fadogia agrestis (Schweinf. Ex Hiern) stem in male rat liver and kidney. Human Exp Toxicol. 2009;28(8):469–78.
Busari MB, Muhammad HL, Ogbadoyi EO, Kabiru AY, Sani S, Yusuf RS. In vivo evaluation of antidiabetic properties of seed oil of Moringa oleifera lam. Journal of applied life sciences international. 2015;2(4):160–74. https://doi.org/10.9734/JALSI/2015/16048.
Yakubu MT, Bilbis LS, Lawal M, Akanji MA. Evaluation of selected parameters of rat liver and kidney function following repeated administration of yohimbine. Biokemistri. 2003;15(2):50–6.
Tietz NW, Burtis CA, Ashwood ER. Tietz textbook of clinical chemistry. Saunders; 1994.
Schoenfeld RG, Lewellan CJ. A colorimetric method for determination of serum chloride. Clin Chem. 1964;10(6):533–9. https://doi.org/10.1093/clinchem/10.6.533.
Rec GSCC. Colorimetric method for serum alkaline phosphatase determination. J Clin Biochem. 1972;10(2):182.
Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28(1):56–63. https://doi.org/10.1093/ajcp/28.1.56.
Mu P, Plummer DT. Introduction to practical biochemistry. Tata McGraw-Hill Education; 2001.
Doumas BT, Watson WA, Biggs HG. Albumin standards and the measurement of serum albumin with bromcresol green. Clin Chim Acts. 1971;31(1):87–96. https://doi.org/10.1016/0009-8981(71)90365-2.
Kaplan A, Chaney AL, Lynch RL, Meites S. Urea nitrogen and urinary ammonia. In: standard methods of clinical chemistry (Vol. 5, pp. 245-256). Elsevier; 1965.
El-Boshy ME, Refaat B, Qasem AH, Khan A, Ghaith M, Almasmoum H, Mahbub A, Almaimani RA. The remedial effect of Thymus vulgaris extracts against lead toxicity-induced oxidative stress, hepatorenal damage, immunosuppression, and haematological disorders in rats. Environ Sci Pollut Res. 2019;26(22):22736–46.
Patridge E, Gareiss P, Kinch MS, Hoyer D. An analysis of FDA-approved drugs: natural products and their derivatives. Drug Discov Today. 2016;21(2):204–7. https://doi.org/10.1016/j.drudis.2015.01.009.
Hwang DF, Wang LC. Effect of taurine on toxicity of cadmium in rats. Toxicology. 2001;167(3):173–80. https://doi.org/10.1016/S0300-483X(01)00472-3.
Zheng J, Qiu G, Zhou Y, Ma K, Cui S. Protective effects of taurine against inflammation and apoptosis in cadmium-induced hepatotoxicity. Research square. 2021:1–15.
Mishra A, Sharma AK, Kumar S, Saxena AK, Pandey AK. Bauhinia variegata leaf extracts exhibit considerable antibacterial, antioxidant, and anticancer activities. Biomed Res Int. 2013:1–10.
Nabil A, Elshemy MM, Asem M, Gomaa HF. Protective effect of DPPD on mercury chloride-induced Hepatorenal toxicity in rats. Journal of Toxicology. 2020;2020:1–7. https://doi.org/10.1155/2020/4127284.
Adaramoye OA, Osaimoje DO, Akinsanya AM, Nneji CM, Fafunso MA, Ademowo OG. Changes in antioxidant status and biochemical indices after acute administration of artemether, artemether-lumefantrine and halofantrine in rats. Basic Clin Pharmacol Toxicol. 2008;102(4):412–8. https://doi.org/10.1111/j.1742-7843.2008.00211.x.
Ali ZY, Atia HA, Ibrahim NH. Possible hepatoprotective potential of Cynara scolymus, Cupressus sempervirens and Eugenia jambolana against paracetamol-induced liver injury: in-vitro and in-vivo evidence. Nat Sci. 2010;10(1):75–86.
El-Zayat EA. Evaluation of purified antigens in haemagglutination test (IHA) for determination of cross reactivities in diagnosis of fascioliasis and schistosomiasis. J Egypt Soc Parasitol. 1996;26(3):677–85.
Shalan MG, Mostafa MS, Hassouna MM, El-Nabi SH, El-Refaie A. Amelioration of lead toxicity on rat liver with vitamin C and silymarin supplements. Toxicology. 2005a;206(1):1–15. https://doi.org/10.1016/j.tox.2004.07.006.
Lakshmi BVS, Sudhakar M, Aparna M. Protective potential of black grapes against lead induced oxidative stress in rats. Environ Toxicol Pharmacol. 2013;35(3):361–8. https://doi.org/10.1016/j.etap.2013.01.008.
Offor SJ, Mbagwu HO, Orisakwe OE. Lead induced hepato-renal damage in male albino rats and effects of activated charcoal. Front Pharmacol. 2017;8:107. https://doi.org/10.3389/fphar.2017.00107.
Akinrinde AS, Oduwole O, Akinrinmade FJ, Bolaji-Alabi FB. Nephroprotective effect of methanol extract of Moringa oleifera leaves on acute kidney injury induced by ischemia-reperfusion in rats. Afr Health Sci. 2020;20(3):1382–96.
Azab EA. Hepatoprotective effect of sesame oil against lead induced liver damage in albino mice: histological and biochemical studies. American Journal of BioScience. 2014;2(6–2):1–11. https://doi.org/10.11648/j.ajbio.s.2014020602.11.
Fadrowski JJ, Navas-Acien A, Tellez-Plaza M, Guallar E, Weaver VM, Furth SL. Blood lead level and kidney function in US adolescents: the third National Health and nutrition examination survey. Arch Intern Med. 2010;170(1):75–82. https://doi.org/10.1001/archinternmed.2009.417.
Barrera C, Valenzuela R, Rincón MÁ, Espinosa A, Echeverria F, Romero N, et al. Molecular mechanisms related to the hepatoprotective effects of antioxidant-rich extra virgin olive oil supplementation in rats subjected to short-term iron administration. Free Radic Biol Med. 2018;126:313–21. https://doi.org/10.1016/j.freeradbiomed.2018.08.030.
Bayram B, Ozcelik B, Grimm S, Roeder T, Schrader C, Ernst IM, et al. A diet rich in olive oil phenolics reduces oxidative stress in the heart of SAMP8 mice by induction of Nrf2-dependent gene expression. Rejuvenation Res. 2012;15(1):71–81. https://doi.org/10.1089/rej.2011.1245.
Martín MA, Ramos S, Granado-Serrano AB, Rodríguez-Ramiro I, Trujillo M, Bravo L, et al. Hydroxytyrosol induces antioxidant/detoxificant enzymes and Nrf2 translocation via extracellular regulated kinases and phosphatidylinositol-3-kinase/protein kinase B pathways in HepG2 cells. Mol Nutr Food Res. 2010;54(7):956–66. https://doi.org/10.1002/mnfr.200900159.
Valenzuela R, Illesca P, Echeverría F, Espinosa A, Rincón-Cervera MÁ, Ortiz M, et al. Molecular adaptations underlying the beneficial effects of hydroxytyrosol in the pathogenic alterations induced by a high-fat diet in mouse liver: PPAR-α and Nrf2 activation, and NF-κB down-regulation. Food Funct. 2017;8(4):1526–37. https://doi.org/10.1039/C7FO00090A.
Zhao B, Ma Y, Xu Z, Wang J, Wang F, Wang D, et al. Hydroxytyrosol, a natural molecule from olive oil, suppresses the growth of human hepatocellular carcinoma cells via inactivating AKT and nuclear factor-kappa B pathways. Cancer Lett. 2014;347(1):79–87. https://doi.org/10.1016/j.canlet.2014.01.028.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
The submitted work was performed in collaboration with all authors. The experiment was designed by MBB, RUH and HLM. RSY, JOA and YOI carried out the laboratory experiment under the supervision of MBB and RUM while FMM, EBB conducted the detail literature review. Critical revision of the article was done by MBB, RUH and HLM. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Busari, M.B., Hamzah, R.U., Muhammad, H.L. et al. Phenolic rich-extracts from Nauclea latifolia fruit restored Lead acetate-induced liver and kidney damaged in Wistar rats. Clin Phytosci 7, 87 (2021). https://doi.org/10.1186/s40816-021-00322-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40816-021-00322-z