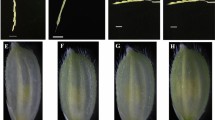
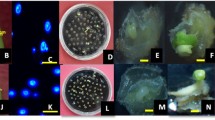
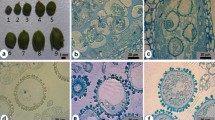
Abstract
Radish (Raphanus sativus L.), an important annual or biennial root vegetable crop, is widely cultivated in the world for its high nutritive value. Isolated microspore culture (IMC) is one of the most effective methods for rapid development of homozygous lines. Due to imperfection of the IMC technology system, it is particularly important to establish an efficient IMC system in radish. In this study, the effects of different factors on radish microspore embryogenesis were investigated with 23 genotypes. Buds with the largest population of late-uninucleate-stage microspores were most suitable for embryogenesis, with a ratio of petal length to anther length (P/A) in buds of about 3/4 ~ 1. Cold pretreatment was found to be genotype specific, and the highest microspore-derived embryoid (MDE) yield occurred for treatment of the heat shock of 48 h. In addition, the supplement of 0.75 g/L activated charcoal (AC) could increase the yield of embryoids. It was found that genotypes, bud size, as well as temperature treatments had significant effects on microspore embryogenesis. Furthermore, somatic embryogenesis–related kinase (SERK) genes were profiled by reverse transcription quantitative polymerase chain reaction (RT-qPCR) analysis, which indicated that they are involved in the process of MDE formation and plantlet regeneration. The ploidy of microspore-derived plants was identified by chromosome counting and flow cytometry, and the microspore-derived plants were further proved as homozygous plants through expressed sequence tags-simple sequence repeats (EST-SSR) and genetic-SSR markers. The results would facilitate generating the large-scale double haploid (DH) from various genotypes, and promoting further highly efficient genetic improvement in radish.





Similar content being viewed by others
Data availability
All data generated or analyzed in this study are included in this published article and its supplementary information files.
Code availability
Not applicable.
References
Ahmadi B, Masoomi-Aladizgeh F, Shariatpanahi ME (2016) Molecular characterization and expression analysis of SERK1 and SERK2 in Brassica napus L.: implication for microspore embryogenesis and plant regeneration. Plant Cell Rep 35(1):1–9. https://doi.org/10.1007/s00299-015-1878-6
Ahmadi B, Shariatpanahi ME, Teixeira da Silva JA (2014a) Efficient induction of microspore embryogenesis using abscisic acid, jasmonic acid and salicylic acid in Brassica napus L. Plant Cell Tiss Org 116:343–351. https://doi.org/10.1007/s11240-013-0408-x
Ahmadi B, Shariatpanahi ME, Ojaghkandi MA (2014b) Plant Cell Tiss Org 118:497–505. https://doi.org/10.1007/s11240-014-0501-9
Bhatia R, Dey SS, Parkash C, Sharma K, Sood S, Kumar R (2018) Modification of important factors for efficient microspore embryogenesis and doubled haploid production in field grown white cabbage (Brassica oleracea var. capitata L.) genotypes in India. Sci Hortic 233:178–187. https://doi.org/10.1016/j.scienta.2018.01.017
Bhatia R, Dey SS, Sood S, Sharma K, Sharma VK, Parkash C, Kumar R (2016) Optimizing protocol for efficient microspore embryogenesis and doubled haploid development in different maturity groups of cauliflower (B. oleracea var. botrytis L.) in India. Euphytica 212:439–454. https://doi.org/10.1007/s10681-016-1775-2
Bhatia R, Dey SS, Sood S, Sharma K, Parkash C, Kumar R (2017) Efficient microspore embryogenesis in cauliflower (Brassica oleracea var. botrytis L.) for development of plants with different ploidy level and their use in breeding programme. Sci Hortic 216:83–92. https://doi.org/10.1016/j.scienta.2016.12.020
Brew-Appiah Rhoda AT, Ankrah N, Liu W, Konzak CF, von Wettstein D, Rustgi S (2013) Generation of doubled haploid transgenic wheat lines by microspore transformation. PLoS ONE 8(11):e80155. https://doi.org/10.1371/journal.pone.0080155
Chen WS, Zhang Y, Ren J, Ma YY, Liu Z, Hui F (2019) Effects of methylene blue on microspore embryogenesis and plant regeneration in ornamental kale (Brassica oleracea var. acephala). Sci Hortic 248:1–7. https://doi.org/10.1016/j.scienta.2018.12.048
Cheng Y, Ma RL, Jiao YS, Qiao N, Li TT (2013) Impact of genotype, plant growth regulators and activated charcoal on embryogenesis induction in microspore culture of pepper (Capsicum annuum L.). S Afr J Bot 88:306–309. https://doi.org/10.1016/j.sajb.2013.08.012
Chun C, Park H, Na H (2011) Microspore-derived embryo formation in radish (Raphanus sativus L.) according to nutritional and environmental conditions. Hortic Environ Biotechnol 52, 530
Corral-Martínez and Seguí-Simarro, 2012 Corral-Martínez P, Seguí-Simarro JM (2012) Efficient production of callus-derived doubled haploids through isolated microspore culture in eggplant (Solanum melongena L.). Euphytica 187(1):47–61 https://doi.org/10.1007/s10681-012-0715-z
Cristea TO (2013) The influence of pH on microspore embryogenesis of white cabbage (Brassica oleracea L). Notulae Scientia Biologicae 54:485–489. https://doi.org/10.15835/nsb549122
Dias JS (1999) Effect of activated charcoal on Brassica oleracea microspore culture embryogenesis. Euphytica 108:65–69. https://doi.org/10.1023/A:1003634030835
Dubas E, MoravčíÍková J, Libantová J, Matušíková I, Benková E, Zur I, Krzewska M (2014) The influence of heat stress on auxin distribution in transgenic B. napus microspores and microspore-derived embryos. Protoplasma 251(5):1077–1087. https://doi.org/10.1007/s00709-014-0616-1
Fan LX, Wang Y, Xu L, Tang MJ, Zhang XL, Ying JL, Li C, Dong JH, Liu LW(2020) A genome-wide association study uncovers a critical role of the RsPAP2 gene in red-skinned Raphanus sativus L. Hortic Res 7:164. https://doi.org/10.1038/s41438-020-00385-y
Gu HH, Zhao ZQ, Sheng XG, Yu HF, Wang JS (2014) Efficient doubled haploid production in microspore culture of loose-curd cauliflower (Brassica oleracea var. botrytis). Euphytica 195:467–475. https://doi.org/10.1007/s10681-013-1008-x
Han N, Kim SU, Park HY, Na H (2014) Microspore-derived embryo formation and morphological changes during the isolated microspore culture of radish (Raphanus sativus L.). Kor J Hort Sci Technol 32(3):382–389. https://doi.org/10.7235/hort.2014.13170
Hecht V, Vielle-Calzada JP, Hartog MV, Schmidt EDL, Boutilier K, Grossniklaus U, de Vries SC (2001) The Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASE 1 gene is expressed in developing ovules and embryos and enhances embryogenic competence in culture. Plant Physiol 127:803–816. https://doi.org/10.1104/pp.010324
Hosp J, Ribarits A, Jin Y, Tashpulatov A, Resch T, Friedmann C, Ankele E, Voronin V, Palme K, Heberle-Bors E, Touraev E (2014) A tobacco homolog of DCN1 is involved in pollen development and embryogenesis. Plant Cell Rep 33(7):1187–1202
Kozar EV, Domblides EA, Soldatenko AV (2020) Factors affecting DH plants in vitro production from microspores of European radish. Vavilovskii Zhurnal Genet Sel 24:31–39
Kozar EV, Domblides EA, Soldatenko AV (2021) Embryogenesis of European radish (Raphanus sativus L. subsp. sativus Convar. Radicula) in culture of isolated microspores in vitro. Plants 10(10):2117. https://doi.org/10.3390/plants10102117
Lichter R (1982) Induction of haploid plants from isolated pollen of Brassica napus L. Z Pflanzenphysiol 105:427–434. https://doi.org/10.1016/S0044-328X(82)80040-8
Lionneton E, Beuret W, Delaitre C, Ochatt S, Rancillac M (2001) Improved microspore culture and doubled-haploid plant regeneration in the brown condiment mustard (Brassica juncea). Plant Cell Rep 20:126–130. https://doi.org/10.1007/s002990000292
Luo XB, Xu L, Liang DY, Wang Y, Zhang W, Zhu XW, Zhu YL, Jiang HY, Tang MJ, Liu LW (2017) Comparative transcriptomics uncovers alternative splicing and molecular marker development in radish (Raphanus sativus L.). BMC genomics 18:505. https://doi.org/10.1186/s12864-017-3874-4
Luo XB, Xu L, Wang Y, Dong JH, Chen YL, Tang MJ, Fan LX, Zhu YL, Liu LW (2020) An ultra-high-density genetic map provides insights into genome synteny, recombination landscape and taproot skin colour in radish (Raphanus sativus L.). Plant Biotechnol J 18:274–286. https://doi.org/10.1111/pbi.13195
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Makowska K, Oleszczuk S, Zimny A, Czaplicki A, Zimny J (2015) Androgenic capability among genotypes of winter and spring barley. Plant Breed 134:668–674. https://doi.org/10.1111/pbr.12312
Mao ZL, Zhang ZC, Yao YM, Dai ZL, QIN WF, Pan YP, (2012) Observation of microspore embryogenesis and ploidy identification of regenerated plants. Acta Botanica Sinica 32(10):2016–2022
Munoz-Amatriain M, Svensson JT, Castillo AM, Close TJ, Valle’s MP (2009) Microspore embryogenesis: assignment of genes to embryo formation and green vs. albino plant production. Funct Integr Genomic 9:311–323. https://doi.org/10.1007/s10142-009-0113-3
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Oleszczuk S, Sowa S, Zimny J (2004) Direct embryogenesis and green plant regeneration from isolated microspores of hexaploid triticale (×Triticosecale Wittmack) cv. Bogo Plant Cell Rep 22:885–893. https://doi.org/10.1007/s00299-004-0796-9
Pilarska M, Malec P, Salaj J, Bartnicki F, Konieczny R (2016) High expression of Somatic Embryogenesis Receptor-like Kinase coincides with initiation of various developmental pathways in vitro culture of Trifolium nigrescens. Protoplasma 253:345–355. https://doi.org/10.1007/s00709-015-0814-5
Podio M, Felitti SA, Siena LA, Delgado L, Mancini M, Seijo JG, Gonza ´lez AM, Pessino SC, Ortiz JP, (2014) Characterization and expression analysis of somatic embryogenesis receptor kinase (SERK) genes in sexual and apomictic Paspalum notatum. Plant Mol Biol 84:479–495. https://doi.org/10.1007/s11103-013-0146-9
Sato S, Katoh N, Iwai S, Hagimori M (2002) Effect of low temperature pretreatment of buds or inflorescence on isolated microspore culture in Brassica rapa (syn. B. campestris). Breed Sci 52:23–26
Segui-Simarro JM, Nuez F (2008) How microspores transform into haploid embryos: changes associated with embryogenesis induction and microspore derived embryogenesis. Physiol Plant 134:1–12
Shariatpanahi ME, Ahmadi B (2016) Isolated microspore culture and its applications in plant breeding and genetics. In: Anis M, Ahmad N (eds) Plant tissue culture: propagation, conservation and crop improvement. Springer, Singapore, pp 487–507. https://doi.org/10.1007/978-981-10-1917-3_21
Shumilina D, Kornyukhin D, Domblides E, Soldatenko A, Artemyeva A (2020) Effects of genotype and culture conditions on microspore embryogenesis and plant regeneration in Brassica Rapa ssp. Rapa l Plants (basel) 9(2):278. https://doi.org/10.3390/plants9020278
Shumilina DV, Shmykova NA, Bondareva LL, Suprunova TP (2015) Effect of genotype and medium culture content on microspore derived embryo formation in Chinese cabbage (Brassica rapa ssp. Chinensis cv. Lastochka). Biol. Bull 42(4):302–309. https://doi.org/10.1134/S1062359015040135
Solís MT, Berenguer E, Risueño MC, Testillano PS (2016) BnPME is progressively induced after microspore reprogramming to embryogenesis, correlating with pectin de-esterifcation and cell differentiation in Brassica napus. BMC Plant Biol 16:176. https://doi.org/10.1186/s12870-016-0863-8
Supena EDJ, Suharsono S, Jacobsen E, Custers JBM (2006) Successful development of a shed-microspore culture protocol for doubled haploid production in Indonesian hot peppers (Capsicum annuum L.). Plant Cell Rep 25:1–10. https://doi.org/10.1007/s00299-005-0028-y
Takahata Y, Komatsu H, Kaizuma N (1996) Microspore culture of radish (Raphanus sativus.L): influence of genotype and culture conditions on embryogenesis. Plant Cell Report 16:163–166. https://doi.org/10.1007/BF01890859
Tang X, Liu Y, He Y, Ma L, Sun MX (2013) Exine dehiscing induces rape microspore polarity, which results in different daughter cell fate and fixes the apical-basal axis of the embryo. Exp Bot 64:215–228. https://doi.org/10.1093/jxb/ers327
Testillano PS (2019) Microspore embryogenesis: targeting the determinant factors of stress-induced cell reprogramming for crop improvement. J Exp Bot 70(11):2965–2978. https://doi.org/10.1093/jxb/ery464
Tsuwamoto R, Fukuoka H, Takahata Y (2007) Identification and characterization of genes expressed in early embryogenesis from microspores of Brassica napus. Planta 225(3):641–652. https://doi.org/10.1007/s00425-006-0388-8
Tuncer B, Cig A, Yanmaz R, Yasar F (2016) Effect of heat shock treatment on microspore embryogenesis in Brassica oleracea Species. Tarim Bilimleri Dergisi 22:548–554. https://doi.org/10.1501/Tarimbil_0000001413
Wang C, Yao Y, Peng L (2013) Study of isolated microspore culture and plant regeneration from embryoid of radish (Raphanus sativus L.). J Anhui Agri Sci 41(27):19–22
Wang WH, Ye GR, Li BY, Yue ZC, Zhong XM (2013b) Isolated microspore culture and plant regeneration in cabbage. Acta Agriculturae Nucleatae Sinica 27(006):715–722
Winarto B, Teixeira DS, Jaime A (2011) Microspore culture protocol for Indonesian Brassica oleracea. Plant Cell Tissue Organ Cult 107:305–315. https://doi.org/10.1007/s11240-011-9981-z
Xiao J, Chen Y (2002) Study on the cytology and morpholgy of pollen development of Brassica vegetable crops. Journal of Changjiang Vegetables S1:107–108
Yu FQ, Liu HL (1995) The effects of donor plant and medium component on embryo yield in Brassica napus. J HuaZhong Agri University 14(4):327–331
Zhai L, Xu L, Wang Y, Cheng H, Chen Y, Gong Y, Liu L (2014) Novel and useful genic-SSR markers from de novo transcriptome sequencing of radish (Raphanus sativus L.). Mol Breeding 33:611–624. https://doi.org/10.1007/s11032-013-9978-x
Zhang L, Wang Q, Wang Y (2020) Development of inbred lines of Raphanus sativus L. var. ‘Xinlimei’ by isolated microspore culture technology. China Vegetables 1(8):53–56
Zhang L, Zheng P (2013) Isolated microspore culture of spring-white radish (Raphanus sativus L. var. longpinnatus Bailey). Northern Horticulture 000(023):31–33
Zhang Z, Zhang S, Zhang W, Zhang H (2007) Induction of tetraploidy of non-heading Chinese cabbage with late-bolting and identification of chromosome configuration. Acta Botan Boreali-Occiden Sin 27(1):0028–0032
Zhou Y, Feng H, Wang CN, Liu RE (2006) Isolated microspore culture and plantlet formation in Chinese cabbage (Brassica campestris ssp.pekinensis). Journal of Shenyang Agricultural University 37(6):816–820
Zhou Z, Gong Y, Wang X, Liu L, Zhang Y (2007) Induction of isolated microspore and optimization of their culture system of different radish varieties. Acta Bot Boreal-Occident Sin 27(1):0033–0038
Żur I, Dubas E, Krzewska M, Kope P, Malage S (2021) Triticale and barley microspore embryogenesis induction requires both reactive oxygen species generation and efficient system of antioxidative defence. Plant Cell Tiss ORG 145:347–366. https://doi.org/10.1007/s11240-021-02012-7
Acknowledgements
The authors thank the anonymous reviewers and the editors for their helpful comments and suggestions on this manuscript.
Funding
This work was partially supported by grants from the National Natural Science Foundation of China (32172579), the Jiangsu Agricultural S&T Innovation Fund [CX(21)2020, CX(18)3067], the earmarked fund for Jiangsu Agricultural Industry Technology System [JATS(2022)], and the Central Agricultural Major Technology Collaborative Extension Plan-Root and Stem Vegetable Project (2020-SJ-047–01-3).
Author information
Authors and Affiliations
Contributions
Wrote first draft: YC, LL. Designed experimental work: YC, LL, YW, LX, and XS. Provided experimental materials: LL, YZ, LZ, and CZ. Analyzed data: YC. Wrote original manuscript: YC, LL. Wrote and edit review: YC, LL. Supervised the whole work: LL. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.



Rights and permissions
About this article
Cite this article
Chen, Y., Wang, Y., Xu, L. et al. Effects of genotype and culture conditions on microspore embryogenesis in radish (Raphanus sativus L.). Mol Breeding 42, 43 (2022). https://doi.org/10.1007/s11032-022-01312-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-022-01312-w