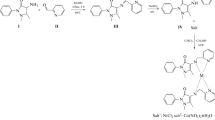
Abstract
Schiff bases are well-known compounds for having significant biological properties. In this study, a new Schiff base ligand and its metal complexes were synthesized, and their antioxidant and enzyme inhibitory activities were evaluated. The new Schiff base ligand was synthesized with the condensation reaction of 6-tert-butyl 3-ethyl 2-amino-4,5-dihydrothieno[2,3-c]pyridine-3,6(7H)-dicarboxylate and 2-hydroxybenzaldehyde compounds. Fe(II), Co(II), and Ni(II) metal complexes of the novel Schiff base ligand were synthesized and characterized. The purity and molecular formula of the synthesized compounds were identified with elemental analysis, infrared, ultraviolet–visible, mass spectrophotometry, powder XRD, magnetic and thermal measurements. The Schiff base acted as a three dentate chelate. The analytical and spectroscopic data suggested an octahedral geometry for the complexes. The in vitro antioxidant method studies elucidated a more effective antioxidant character of the Schiff base ligand than its metal complexes but a less effective antioxidant potential than the standard antioxidant compounds. The enzyme inhibition potentials of the synthesized compounds for AChE, BChE, and GST enzymes were determined by in vitro enzyme activity methods. The Schiff base ligand was discovered to be the best inhibitor for the AChE and BChE with the values of 7.13 ± 0.84 µM and 5.75 ± 1.03 µM Ki, respectively. Moreover, the Fe(II) complex displayed the best Ki value as 9.37 ± 1.06 µM for the GST enzyme. Finally, molecular docking studies were carried out to see the structural interactions of the compounds. The metal complexes demonstrated better binding affinities with the AChE, BChE, and GST enzymes than the Schiff base ligand. This study identified a potential Schiff base molecule against both AChE and BChE targets to further investigate for in vivo and safety evaluation.
Graphical abstract








Similar content being viewed by others
References
Mohapatra RK, Das PK, Pradhan MK, Maihub AA, El-ajaily MM (2018) Biological aspects of Schiff base-metal complexes derived from benzaldehydes: an overview. J Iran Chem Soc 15:2193–2227. https://doi.org/10.1007/s13738-018-1411-2
Laziz D, Beghidja C, Baali N, Zouchoune B, Beghidja A (2019) Synthesis, structural characterization, DFT calculations and biological properties of mono-and dinuclear nickel complexes with tetradentate transformed ligands by aerobic oxidative-coupling reactions. Inorg Chim Acta 497:119085. https://doi.org/10.1016/j.ica.2019.119085
Omar M, Mohamed GG, Ibrahim AA (2009) Spectroscopic characterization of metal complexes of novel Schiff base. Synthesis, thermal and biological activity studies. Spectrochim Acta Part A 73:358–369. https://doi.org/10.1016/j.saa.2009.02.043
Mukwevho E, Ferreira Z, Ayeleso A (2014) Potential role of sulfur-containing antioxidant systems in highly oxidative environments. Molecules 19:19376–19389. https://doi.org/10.3390/molecules191219376S
Kundu S, Pramanik AK, Mondal AS, Mondal TK (2016) Ni(II) and Pd(II) complexes with new N, O donor thiophene appended Schiff base ligand: Synthesis, electrochemistry, X-ray structure and DFT calculation. J Mol Struct 1116:1–8. https://doi.org/10.1016/j.molstruc.2016.03.013
Yousif E, Majeed A, Al-Sammarrae K, Salih N, Salimon J, Abdullah B (2017) Metal complexes of Schiff base: preparation, characterization and antibacterial activity. Arab J Chem 10:S1639–S1644. https://doi.org/10.1016/j.arabjc.2013.06.006
Ramalingan C, Balasubramanian S, Kabilan S, Vasudevan M (2004) Synthesis and study of antibacterial and antifungal activities of novel 1-[2-(benzoxazol-2-yl)ethoxy]-2,6-diarylpiperidin-4-ones. Eur J Med Chem 39:527–533. https://doi.org/10.1016/j.ejmech.2004.02.005
Ambika S, Manojkumar Y, Arunachalam S, Gowdhami B, Sundaram KKM, Solomon RV, Venuvanalingam P, Akbarsha MA, Sundararaman M (2019) Biomolecular interaction, anti-cancer and anti-angiogenic properties of cobalt(III) Schiff base complexes. Sci Rep 9:1–14. https://doi.org/10.1038/s41598-019-39179-1
Buldurun K, Turan N, Aras A, Mantarcı A, Turkan F, Bursal E (2019) Spectroscopic and structural characterization, enzyme inhibitions, and antioxidant effects of new Ru(II) and Ni(II) complexes of Schiff base. Chem Biodivers 16:e1900243. https://doi.org/10.1002/cbdv.201900243
Jain P, Pandey G, Kumar D, Chandra S (2019) Prospects of biologically active Schiff’s base ligand and metal complexes in drug discovery. Adv Sci Eng Med 11:144–154. https://doi.org/10.1166/asem.2019.2302
Liu X, Hamon J-R (2019) Recent developments in penta-, hexa- and heptadentate Schiff base ligands and their metal complexes. Coord Chem Rev 389:94–118. https://doi.org/10.1016/j.ccr.2019.03.010
Yamada T, Fujii T, Kanai T, Amo T, Imanaka T, Nishimasu H, Wakagi T, Shoun H, Kamekura M, Kamagata Y (2005) Expression of acetylcholine (ACh) and ACh-synthesizing activity in Archaea. Life Sci 77:1935–1944. https://doi.org/10.1016/j.lfs.2005.01.026
Turkan F, Cetin A, Taslimi P, Karaman HS, Gulçin İ (2019) Synthesis, characterization, molecular docking and biological activities of novel pyrazoline derivatives. Arch Pharm 352:1800359. https://doi.org/10.1002/ardp.201800359
Cheng Z-Q, Zhu K-K, Zhang J, Song J-L, Muehlmann LA, Jiang C-S, Liu C-L, Zhang H (2019) Molecular-docking-guided design and synthesis of new IAA-tacrine hybrids as multifunctional AChE/BChE inhibitors. Bioorg Chem 83:277–288. https://doi.org/10.1016/j.bioorg.2018.10.057
Ma W, Bi J, Zhao C, Gao Y, Zhang G (2020) Design, synthesis and biological evaluation of acridone glycosides as selective BChE inhibitors. Carbohydr Res 491:107977. https://doi.org/10.1016/j.carres.2020.107977
Turan N, Buldurun K, Adiguzel R, Aras A, Turkan F, Bursal E (2021) Investigation of spectroscopic, thermal, and biological properties of FeII, CoII, ZnII, and RuII complexes derived from azo dye ligand. J Mol Struct 1244:130989. https://doi.org/10.1016/j.molstruc.2021.130989
Zhang K, Wong KP, Chow P (2003) Conjugation of chlorambucil with GSH by GST purified from human colon adenocarcinoma cells and its inhibition by plant polyphenols. Life Sci 72:2629–2640. https://doi.org/10.1016/s0024-3205(03)00173-5
Türkan F, Huyut Z, Taslimi P, Huyut MT, Gülçin İ (2020) Investigation of the effects of cephalosporin antibiotics on glutathione S-transferase activity in different tissues of rats in vivo conditions in order to drug development research. Drug ChemToxicol 43:423–428. https://doi.org/10.1080/01480545.2018.1497644
Gulcin İ (2020) Antioxidants and antioxidant methods: an updated overview. Archi Toxicol 94:651–715. https://doi.org/10.1007/s00204-020-02689-3
Özdemir M, Buldurun K (2020) Synthesis, characterization and catalytic activities of the new Schiff base ligands and their Ru(II), Pd(II) metal complexes, The Graduate School of Natural and Applied Science of Muş Alparslan University. Thesis of Master of Science in Chemistry Science.
Aras A, Bursal E, Alan Y, Turkan F, Alkan H, Kılıç Ö (2018) Polyphenolic content, antioxidant potential and antimicrobial activity of Satureja boissieri. Iran J Chem Chem Eng 37:209–219. https://doi.org/10.30492/IJCCE.2018.32113
Aras A, Bursal E, Türkan F, Tohma H, Kılıç Ö, Gülçin İ, Köksal E (2019) Phytochemical content, antidiabetic, anticholinergic, and antioxidant activities of endemic Lecokia cretica extracts. Chem Biodivers 16:e1900341. https://doi.org/10.1002/cbdv.201900341
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139. https://intl.jbc.org/cgi/content/abstract/249/22/7130
Trott O, Olson AJ (2009) AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 3:55–461. https://doi.org/10.1002/jcc.21334
Hanwell MD, Curtis DE, Lonie DC, Vandermeerschd T, Zurek E, Hutchison GR (2012) Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J Cheminform 4:17. https://doi.org/10.1186/1758-2946-4-17
Pahonțu E, Proks M, Shova S, Lupașcu G, Ilieș DC, Bărbuceanu Ștefania F, Socea LI, Badea M, Păunescu V, Istrati D, Gulea A, Drăgănescu D, Pîrvu CED (2019) Synthesis, characterization, molecular docking studies and in vitro screening of new metal complexes with Schiff base as antimicrobial and antiproliferative agents. Appl Organomet Chem 33:e5185. https://doi.org/10.1002/aoc.5185
The PyMOL Molecular Graphics System, Version 2.4 Schrödinger, LLC (2020)
Discovery Studio Visualizer, Dassault Systèmes BIOVIA, San Diego (2020)
Vinusha HM, Kollur SP, Revanasiddappa HD, Ramu R, Shirahatti PS, Prasad MNN, Chandrashekar S, Begum M (2019) Preparation, spectral characterization and biological applications of Schiff base ligand and its transition metal complexes. Results Chem 1:100012. https://doi.org/10.1016/j.rechem.2019.100012
Mahmoud WH, Deghadi RG, Mohamed GG (2020) Metal complexes of ferrocenyl-substituted Schiff base: preparation, characterization, molecular structure, molecular docking studies, and biological investigation. J Organomet Chem 917:121113. https://doi.org/10.1016/j.jorganchem.2020.121113
El-Behery M, El-Twigry H (2007) Synthesis, magnetic, spectral, and antimicrobial studies of Cu(II), Ni(II), Co(II), Fe(III), and UO2(II) complexes of a new Schiff base hydrazone derived from 7-chloro-4-hydrazinoquinoline. Spectrochim Acta Part A 66:28–36. https://doi.org/10.1016/j.saa.2006.02.017
Natarajan C, Sheela C, Athappan P (1990) Synthesis and study of cobalt(II), nickel(II), copper(II) and zinc(II) complexes of 2-formyl- and 2-acetyl-cyclohexanones. Indian J Chem 29A:569–572. http://nopr.niscair.res.in/handle/123456789/46384
Bera P, Aher A, Brandao P, Manna SK, Mondal G, Jana A, Santra A, Jana H, Bera P (2020) vInduced apoptosis against U937 cancer cells by Fe(II), Co(III) and Ni(II) complexes with a pyrazine-thiazole ligand: synthesis, structure and biological evaluation. Polyhedron 182:114503. https://doi.org/10.1016/j.poly.2020.114503
Abdel-Rahman LH, El-Khatib RM, Nassr LAE, Abu-Dief AM, Lashin FE-D (2013) Design, characterization, teratogenicity testing, antibacterial, antifungal and DNA interaction of few high spin Fe(II) Schiff base amino acid complexes. Spectrochim Acta Part A 111:266–276. https://doi.org/10.1016/j.saa.2013.03.061
Houghton DT, Gydesen NW, Arulsamy N, Mehn MP (2010) Synthesis and characterization of iron(II) quinaldate complexes. Inorg Chem 49:879–887. https://doi.org/10.1021/ic901464b
Turan N, Buldurun K, Alan Y, Savci A, Çolak N, Mantarcı A (2019) Synthesis, characterization, antioxidant, antimicrobial and DNA binding properties of ruthenium(II), cobalt(II) and nickel(II) complexes of Schiff base containing o-vanillin. Res Chem Intermed 45:3525–3540. https://doi.org/10.1007/s11164-019-03806-3
Gönül İ (2019) Synthesis and structural characterization of ONO type tridentate ligands and their Co(II) and Ni(II) complexes: Investigation of electrical conductivity and antioxidant properties. Inorganica Chim Acta 495:119027. https://doi.org/10.1016/j.ica.2019.119027
Nithya P, Rajamanikandan R, Simpson J, Ilanchelian M, Govindarajan S (2018) Solvent assisted synthesis, structural characterization and biological evaluation of cobalt(II) and nickel(II) complexes of Schiff bases generated from benzyl carbazate and cyclic ketones. Polyhedron 145:200–217. https://doi.org/10.1016/j.poly.2018.02.008
Buldurun K, Turan N, Bursal E, Mantarcı A, Turkan F, Taslimi P, Gülçin İ (2020) Synthesis, spectroscopic properties, crystal structures, antioxidant activities and enzyme inhibition determination of Co(II) and Fe(II) complexes of Schiff base. Res Chem Intermed 46:283–297. https://doi.org/10.1007/s11164-019-03949-3
Bingöl M, Turan N (2020) Schiff base and metal(II) complexes containing thiophene-3-carboxylate: synthesis, characterization and antioxidant activities. J Mol Struct 1205:127542. https://doi.org/10.1016/j.molstruc.2019.127542
Keypour H, Ansari N, Mahmoudabadi M, Karamian R, Farida SHM, Moghadam ME, Gable RW (2020) Mn(III), Zn(II) and Pt(II) macroacyclic complexes: Synthesis, X-ray structures, anticancer and antioxidant activities. Inorganica Chim Acta 509:119705. https://doi.org/10.1016/j.ica.2020.119705
El-Medani SM, Makhlouf AA, Moustafa H, Afifi MA, Haukka M, Ramadan RM (2020) Spectroscopic, crystal structural, theoretical and biological studies of phenylacetohydrazide Schiff base derivatives and their copper complexes. J Mol Struct 1208:127860. https://doi.org/10.1016/j.molstruc.2020.127860
Larik FA, Saeed A, Faisal M, Hamdani S, Jabeen F, Channar PA, Mumtaz A, Khan I, Kazi MA, Abbas Q (2020) Synthesis, inhibition studies against AChE and BChE, drug-like profiling, kinetic analysis and molecular docking studies of N-(4-phenyl-3-aroyl-2(3H)-ylidene) substituted acetamides. J Mol Struct 1203:127459. https://doi.org/10.1016/j.molstruc.2019.127459
El-Sayed NAE, Farag AES, Ezzat MAF, Akincioglu H, Gülçin İ, Abou-Seri SM (2019) Design, synthesis, in vitro and in vivo evaluation of novel pyrrolizine-based compounds with potential activity as cholinesterase inhibitors and anti-Alzheimer’s agents. Bioorg Chem 93:103312. https://doi.org/10.1016/j.bioorg.2019.103312
Faisal M, Ahmed M, Hussain S, Larik FA, Saeed A (2019) Investigating the effectiveness of classical and eco-friendly approaches for synthesis of dialdehydes from organic dihalides. Green Process Synth 8:635–648. https://doi.org/10.1515/gps-2019-0034
Lv PC, Wang KR, Yang Y, Mao WJ, Chen J, Xiong J, Zhu HL (2009) Design, synthesis and biological evaluation of novel thiazole derivatives as potent FabH inhibitors. Bioorg Med Chem Lett 19:6750–6754. https://doi.org/10.1016/j.bmcl.2009.09.111
Bursal E, Yilmaz MA, Izol E, Türkan F, Atalar MN, Murahari M, Aras A, Ahmad M (2021) Enzyme inhibitory function and phytochemical profile of Inula discoidea using in vitro and in silico methods. Biophys Chem. https://doi.org/10.1016/j.bpc.2021.106629
Rubino FM (2015) Toxicity of glutathione-binding metals: a review of targets and mechanisms. Toxics 3:20–62. https://doi.org/10.3390/toxics3010020
Balcı N, Türkan F, Şakiroğlu H, Aygün A, Şen F (2019) Purification and characterization of glutathione S-transferase from blueberry fruits (Vaccinium arctostaphylos L.) and investigated of some pesticide inhibition effects on enzyme activity. Heliyon 5:e01422. https://doi.org/10.1016/j.heliyon.2019.e01422
Bursal E, Turkan F, Buldurun K, Turan N, Aras A, Çolak N, Murahari M, Yergeri MC (2021) Transition metal complexes of a multidentate Schiff base ligand containing pyridine: synthesis, characterization, enzyme inhibitions, antioxidant properties, and molecular docking studies. Biometals 34(2):393–406. https://doi.org/10.1007/s10534-021-00287-z
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that there is no conflict of interest regarding the publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Turan, N., Buldurun, K., Türkan, F. et al. Some metal chelates with Schiff base ligand: synthesis, structure elucidation, thermal behavior, XRD evaluation, antioxidant activity, enzyme inhibition, and molecular docking studies. Mol Divers 26, 2459–2472 (2022). https://doi.org/10.1007/s11030-021-10344-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-021-10344-x