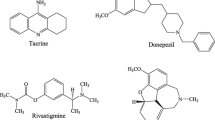
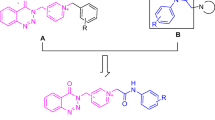
Abstract
A new series of compounds based on benzodiazepine-1,2,3-triazole were synthesized and evaluated as cholinesterase inhibitors by Ellman’s method. The compounds proved to be selective inhibitors of butyrylcholinesterase (BuChE) over acetylcholinesterase. The most potent compound was 3,3-dimethyl-11-(3-((1-(4-nitrobenzyl)-1H-1,2,3-triazol-4-yl)methoxy)phenyl)-2,3,4,5,10,11-hexahydro-1H-dibenzo[b,e][1,4]diazepin-1-one, identified as a submicromolar inhibitor of BuChE with IC50 value of 0.2 µM. In addition, the amyloid-β self-aggregation evaluation studies for selected compounds showed potent inhibitory effects compared to donepezil. The docking and cell viability studies supported the potential of compound 9b-6 as significant BuChE inhibitor.
Graphic abstract







Similar content being viewed by others
References
Reitz C, Mayeux R (2015) Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol 88:640–651. https://doi.org/10.1016/j.bcp.2013.12.024
Mayeux R, Stern Y (2012) Epidemiology of alzheimer disease. Cold Spring Harb Perspect Med 2:1–18. https://doi.org/10.1101/cshperspect.a006239
Reitz C, Brayne C, Mayeux R (2012) Epidemiology of Alzheimer disease. Nat Rev Neurol 7:137–152. https://doi.org/10.1038/nrneurol.2011.2
Korolev IO (2014) Alzheimer’s disease: a clinical and basic science review. Med Stud Res J 4:24–33
Burns A, Iliffe S (2009) Alzheimer’s disease. BMJ 338:467–471. https://doi.org/10.1136/bmj.b158
Scheltens P, Blennow K, Breteler MM, De Strooper B, Frisoni GB, Salloway S, Van der Flier WM (2016) Alzheimer’s disease. Lancet 30:505–517. https://doi.org/10.1016/S0140-6736(15)01124-1
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297:353–356. https://doi.org/10.1126/science.1072994
Bulic B, Pickhardt M, Mandelkow E (2013) Progress and developments in tau aggregation inhibitors for alzheimer disease. J Med Chem 56:4135–4155. https://doi.org/10.1021/jm3017317
Hooli B, Tanzi RE (2016) The genetic basis of Alzheimer’s disease. In: Wolfe MS (ed) Developing therapeutics for Alzheimer’s disease. Academic Press, Cambridge, pp 23–37. https://doi.org/10.1016/b978-0-12-802173-6.00002-2
Buckley JS, Salpeter SR (2015) A risk-benefit assessment of dementia medications: systematic review of the evidence. Drugs Aging 32:453–467. https://doi.org/10.1007/s40266-015-0266-9
Birks J (2006) Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev 1:CD005593. https://doi.org/10.1002/14651858.cd005593
Martorana A, Esposito Z, Koch G (2010) Beyond the cholinergic hypothesis: do current drugs work in Alzheimer’s disease? CNS Neurosci Ther 16:235–245. https://doi.org/10.1111/j.1755-5949.2010.00175.x
Lane RM, Potkin SG, Enz A (2006) Targeting acetylcholinesterase and butyrylcholinesterase in dementia. Int J Neuropsychopharmacol 9:101–124. https://doi.org/10.1017/S1461145705005833
Lockridge O (2015) Review of human butyrylcholinesterase structure, function, genetic variants, history of use in the clinic, and potential therapeutic uses. Pharmacol Ther 148:34–46. https://doi.org/10.1016/j.pharmthera.2014.11.011
Contestabile A (2011) The history of the cholinergic hypothesis. Behav Brain Res 221:334–340. https://doi.org/10.1016/j.bbr.2009.12.044
Schmitz A (2016) Benzodiazepine use, misuse, and abuse: a review. Ment Health Clin 6:120–126. https://doi.org/10.9740/mhc.2016.05.120
Schetinger MRC, Porto NM, Moretto MB, Morsch VM, Da Rocha JBT, Vieira V, Moro F, Neis RT, Bittencourt S, Bonacorso HG et al (2000) New benzodiazepines alter acetylcholinesterase and ATPDase activities. Neurochem Res 25:949–955. https://doi.org/10.1023/A:1007500424392
Shweta V, Sushil K (2017) A mini review on synthetic approaches and biological activities of benzodiazepines. Mini Rev Org Chem 14:453–468. https://doi.org/10.2174/1570193X14666170511121927
Kuno F, Otoguro K, Shiomi K, Iwai Y, Omura S (1996) Arisugacins A and B, novel and selective acetylcholinesterase inhibitors from Penicillium sp. FO-4259. J Antibiot Tokyo 49:742–747
Mohamed L (2012) Design and synthesis of novel 1,4-benzodiazepine derivatives and their biological evaluation as cholinesterase inhibitors. Arch Pharm Res 35:1369–1377. https://doi.org/10.1007/s12272-012-0806-3
Tiwari VK, Mishra BB, Mishra KB, Mishra N, Singh AS, Chen X (2016) Cu-catalyzed click reaction in carbohydrate chemistry. Chem Rev 116:3086–3240. https://doi.org/10.1021/acs.chemrev.5b00408
Kolb HC, Finn MG, Sharpless KB (2001) Click chemistry: diverse chemical function from a few good reactions. Angew Chem Int Ed 40:2004–2021. https://doi.org/10.1002/1521-3773(20010601)40:11%3c2004:AID-ANIE2004%3e3.0.CO;2-5
Meldal M, Tornoe CW (2008) Cu-catalyzed azide-alkyne cycloaddition. Chem Rev 108:2952–3015. https://doi.org/10.1021/cr0783479
Mohammadi-Khanaposhtani M, Saeedi M, Zafarghandi NS, Mahdavi M, Sabourian R, Razkenari EK, Alinezhad H, Khanavi M, Foroumadi A, Shafiee A, Akbarzadeh T (2015) Potent acetylcholinesterase inhibitors: design, synthesis, biological evaluation, and docking study of acridone linked to 1,2,3-triazole derivatives. Eur J Med Chem 6:799–806. https://doi.org/10.1016/j.ejmech.2015.01.044
Li JC, Zhang J, Rodrigues MC, Ding DJ, Longo JP, Azevedo RB, Muehlmann LA, Jiang CS (2016) Synthesis and evaluation of novel 1,2,3-triazole-based acetylcholinesterase inhibitors with neuroprotective activity. Bioorg Med Chem Lett 26:3881–3885. https://doi.org/10.1016/j.bmcl.2016.07.017
Wu G, Gao Y, Kang D, Huang B, Huo Z, Liu H, Poongavanam V, Zhan P, Liu X (2018) Design, synthesis and biological evaluation of tacrine-1,2,3-triazole derivatives as potent cholinesterase inhibitors. Med Chem Commun 9:149–159. https://doi.org/10.1039/C7MD00457E
Wang C, Ikhlef D, Kahlal S, Saillar J-Y, Astruc D (2016) Metal-catalyzed azide-alkyne “click” reactions: mechanistic overview and recent trends. Coord Chem Rev 316:1–20. https://doi.org/10.1016/j.ccr.2016.02.010
Mahdavi M, Lijan H, Bahadorikhalili S, Ma’mani L, Ranjbar PR, Shafiee A (2016) Copper supported β-cyclodextrin grafted magnetic nanoparticles as an efficient recyclable catalyst for one-pot synthesis of 1-benzyl-1H-1,2,3-triazoldibenzodiazepinone derivatives via click reaction. RSC Adv 6:28838–28843
Xu M, Peng Y, Zhu L, Wang S, Ji KJ, Rakesh P (2019) Triazole derivatives as inhibitors of Alzheimer’s disease: current developments and structure-activity relationships. Eur J Med Chem 180:656–672. https://doi.org/10.1016/j.ejmech.2019.07.059
Agalave SG, Maujan SR, Pore VS (2011) Click chemistry: 1,2,3-triazoles as pharmacophores. Chem Asian J 6:2696–2718. https://doi.org/10.1002/asia.20110043
Wang X, Huang B, Liu X, Zhan P (2016) Discovery of bioactive molecules from CuAAC click-chemistry-based combinatorial libraries. Drug Discov Today 21:118–132. https://doi.org/10.1016/j.drudis.2015.08.004
Bozorova K, Zhaoa J, Haji A (2019) 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: a recent Overview. Bioorg Med Chem 27:3511–3531. https://doi.org/10.1016/j.bmc.2019.07.005
Jalili-Baleh L, Nadri H, Forootanfar H, Samzadeh-Kermani A, Küçükkılınç TT, Ayazgok B, Rahimifard M, Baeeri M, Doostmohammadi M, Firoozpour L et al (2018) Novel 3-phenylcoumarin–lipoic acid conjugates as multi-functional agents for potential treatment of Alzheimer’s disease. Bioorg Chem 79:223–234. https://doi.org/10.1016/j.bioorg.2018.04.030
Abedinifar F, Farnia SMF, Mahdavi M, Nadri H, Moradi A, Ghasemi JB, Küçükkılınç TT, Firoozpour L, Foroumadi A (2018) Synthesis and cholinesterase inhibitory activity of new 2-benzofuran carboxamide-benzylpyridinum salts. Bioorg Chem 80:180–188. https://doi.org/10.1016/j.bioorg.2018.06.006
Salehi N, Mirjalili BBF, Nadri H, Abdolahi Z, Forootanfar H, Samzadeh-Kermani A, Küçükkılınç TT, Ayazgok B, Emami S, Haririan I, Sharifzadeh M, Foroumadi A et al (2019) Synthesis and biological evaluation of new N-benzylpyridinium-based benzoheterocycles as potential anti-Alzheimer’s agents. Bioorg Chem 83:559–568. https://doi.org/10.1016/j.bioorg.2018.11.010
Moradi A, Faraji L, Nadri H, Hasanpour Z, Moghadam FH, Pakseresht B, Golshani M, Moghimi S, Ramazani A, Firoozpour L et al (2018) Synthesis, docking study, and biological evaluation of novel umbellipherone/hymecromone derivatives as acetylcholinesterase/butyrylcholinesterase inhibitors. Med Chem Res 27:1741–1747. https://doi.org/10.1007/s00044-018-2187-8
Pouramiri B, Moghimi S, Mahdavi M, Nadri H, Moradi A, Tavakolinejad-Kermani E, Firoozpour L, Asadipour A, Foroumadi A (2017) Synthesis and anticholinesterase activity of new substituted benzo[d]oxazole-based derivatives. Chem Biol Drug Des 89:783–789. https://doi.org/10.1111/cbdd.12902
Asif M (2016) Biological potentials of biological active triazole derivatives: a short review. Org Chem Curr Res 5:2–9. https://doi.org/10.4172/2161-0401.1000173
McGowan D, Nyanguile O, Cummings MD, Vendeville S, Vandyck K, Van den Broeck W, Boutton CW, De Bondt H, Quirynen L, Amssoms K et al (2009) 1,5-Benzodiazepine inhibitors of HCV NS5B polymerase. Bioorg Med Chem Lett 19:2492–2496. https://doi.org/10.1016/j.bmcl.2009.03.035
Vatolina NA, Andin AN (2011) Reactions of dimedone-β-benzoylacrylic acid adduct with nitrogen-containing binucleophiles. Russ J Org Chem 47:408–411. https://doi.org/10.1134/S1070428011030146
Albuquerque HMT, Santos CMM, Cavaleiro JAS, Silva AMS (2018) First intramolecular Diels–Alder reactions using chromone derivatives: synthesis of chromeno[3,4-b]xanthones and 2-(benzo[c]chromenyl)chromones. New J Chem 42:4251–4260. https://doi.org/10.1039/C7NJ05185A
Yesilgul N, Seven O, Guliyev R, Akkaya EU (2018) Energy harvesting in a bodipy-functionalized rotaxane. J Org Chem 83:13228–13232. https://doi.org/10.1021/acs.joc.8b01928
Hans RH, Guantai EM, Lategan C, Smith PJ, Wan B, Franzblau SG, Gut J, Rosenthal PJ, Chibale K (2010) Synthesis, antimalarial and antitubercular activity of acetylenic chalcones. Bioorg Med Chem Lett 20:942–944. https://doi.org/10.1016/j.bmcl.2009.12.062
Ellman GL, Courtney KD, Andresjr V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95. https://doi.org/10.1016/0006-2952(61)90145-9
O’Boyle NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR (2011) Open Babel: an open chemical toolbox. J Cheminform 3:33. https://doi.org/10.1186/1758-2946-3-33
Morris GM, Huey R, Lindstrom W, Sanner M, Belew RK, Goodsell DS, Olson AJ (2009) Autodock4 and Auto DockTools4: automated docking with selective receptor flexibility. J Comput Chem 16:2785–2791. https://doi.org/10.1002/jcc.21256
Levine H (1993) Thioflavine T interaction with synthetic Alzheimer’s disease β-amyloid peptides: detection of amyloid aggregation in solution. Protein Sci 2:404–410. https://doi.org/10.1002/pro.5560020312
Mandegary A, Soodi M, Sharififar F, Ahmadi S (2014) Anticholinesterase, antioxidant, and Neuroprotective effects of Tripleurospermum disciforme and Dracocephalum multicaule. J Ayurveda Integr Med 5:162–166. https://doi.org/10.4103/0975-9476.140474
Acknowledgements
This work was supported and funded by Tehran University of Medical Sciences (TUMS); Grant No. 96-01-92-34801.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mehrazar, M., Hassankalhori, M., Toolabi, M. et al. Design and synthesis of benzodiazepine-1,2,3-triazole hybrid derivatives as selective butyrylcholinesterase inhibitors. Mol Divers 24, 997–1013 (2020). https://doi.org/10.1007/s11030-019-10008-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-019-10008-x