Abstract
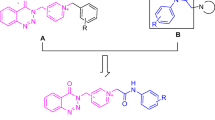
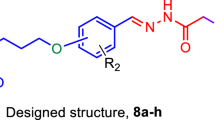
Inhibition of cholinesterases is an effective method to curb Alzheimer’s disease, a progressive and fatal neurological disorder. A series of 6-substituted-3(2H)-pyridazinone-2-acetyl-2-(p-substituted benzalhidrazone) derivatives were designed, synthesized, and their inhibitory effects on acetylcholinesterase and butyrylcholinesterase were evaluated in pursuit of potent dual inhibitors. We obtained our compounds by the reaction of various substituted/nonsubstituted benzaldehydes with 6-[4-(3,4-dichlorophenyl)piperazine-1-yl]-3(2H)-pyridazinone-2-yl acetohydrazide and determined their anticholinesterase activities according to the Ellman’s method. 5f and 5i showed 75.52 and 71.72% acetylcholinesterase inhibition at 100 µg/ml, respectively. 5h and 5f, on the other hand, were the best butyrylcholinesterase inhibitors with 67.16 and 62.03% inhibition at the same concentration, respectively. 5f emerged as a potent dual cholinesterase inhibitor. Through molecular docking studies we predicted the inhibition mechanism of 5f for both enzymes in comparison with our previous derivatives, which differ in inhibition potency, and tried to get insights into the factors that affect receptor affinity in molecular level.







Similar content being viewed by others
References
Abd El-Ghaffar NF, Mohamed MK, Kadah MS, Radwan AM, Said GH, Abd el Al SN (2011) Synthesis and anti-tumor activities of some new pyridazinones containing the 2-phenyl-1H-indolyl moiety. J Chem Pharm Res 3:248–259
Anand P, Singh P (2013) A review on cholinesterase inhibitors for Alzheimer’s disease. Arch Pharm Res 36:375–399
Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E (2011) Alzheimer’s disease. The Lancet 377:1019–1031
Banks JL, Beard HS, Cao Y, Cho AE, Damm W, Farid R, Felts AK, Halgren TA, Mainz DT, Maple JR (2005) Maple, Integrated modeling program, applied chemical theory (IMPACT). J Comput Chem 26:1752–1780
Belluti F, Bartolini M, Bottegoni G, Bisi A, Cavalli A, Andrisano V, Rampa A (2011) Benzophenone-based derivatives: a novel series of potent and selective dual inhibitors of acetylcholinesterase and acetylcholinesterase-induced betaamyloid aggregation. Eur J Med Chem 46:1682–1693
Bentley P, Drive J, Dolan RJ (2011) Cholinergic modulation of cognition: insights from human pharmacological functional neuroimaging. Prog Neurobiol 94:360–388
Bourne Y, Taylor P, Radić Z, Marchot P (2003) Structural insights into ligand interactions at the acetylcholinesterase peripheral anionic site. EMBO J 22:1–12
Brus B, Kosak U, Turk S, Pislar A, Coquelle N, Kos J, Stojan J, Colletier JP, Gobec S (2014) Discovery, biological evaluation, and crystal structure of a novel nanomolar selective butyrylcholinesterase inhibitor. J Med Chem 57:8167–8179
Cacabelos R (2008) Pharmacogenomics in Alzheimer’s disease. Mol Biol 448:213–353
Camps P, Formosa X, Galdeano C, Gomez T, Munoz-Torrero D, Scarpellini M, Viayna E, Badia A, Clos MV, Camins A, Pallas M, Bartolini M, Mancini F, Andrisano V, Estelrich J, Lizondo M, Bidon-Chanal A, Luque FJ (2008) Novel donepezil-based inhibitors of acetyl- and butyrylcholinesterase and acetylcholinesterase-induced beta-amyloid aggregation. J Med Chem 51:3588–3598
Camps P, Formosa X, Galdeano C, Gomez T, Munoz-Torrero D, Scarpellini M, Viyana E, Badia A, Clos VM, Camins A, Pallas M, Bartolini M, Mancini F, Andrisano V, Estelrich J, Lizondo M, Bidon-Chanal A, Luque FJ (2009) Novel donepezil-based inhibitors of acetyl- and butyrylcholinesterase and acetylcholinesterase-induced β-amyloid aggregation. J Med Chem 51:3588–3598
Candeias E, Duarte AI, Carvalho C, Correia SC, Cardoso S, Santos RX, Plácido AI, Perry G, Moreira PI (2012) The impairment of insulin signaling in Alzheimer’s disease. IUBMB Life 64(12):951–957
Catto M, Berezin AA, Lo Re D, Loizou G, Demetriades M, De Stradis A, Campagna F, Koutentis PA, Carotti A (2012) Design, synthesis and biological evaluation of benzo[e][1,2,4]triazin-7(1H)-one and [1,2,4]-triazino[5,6,1-jk]carbazol-6-one derivatives as dual inhibitors of beta-amyloid aggregation and acetyl/butyryl cholinesterase. Eur J Med Chem 58:84–97
Chen Y, Su J, Fang L, Liu M, Peng s, Liao H, Lehmann J, Zhang Y (2012) Tacrineferulic acid-nitric oxide (NO) donor trihybrids as potent, multifunctional acetyl and butyrylcholinesterase inhibitors. J Med Chem 55:4309–4321
Cheung J, Rudolph MJ, Burshteyn F, Cassidy MS, Gary EN, Love J, Franklin MC, Height JJ (2012) Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J Med Chem 55:10282–10286
Chiou SY, Huang CF, Hwang MT, Lin G (2009) Comparison of active sites of butyrylcholinesterase and acetylcholinesterase based on inhibition by geometric isomers of benzene-di-N-substituted carbamates. Mol Toxicol 23:303–308
Costanzo P, Cariati L, Desiderio D, Sgammato R, Lamberti A, Arcone R, Salerno R, Nardi M, Masullo M, Oliverio M (2016) Design, synthesis, and evaluation of donepezil-like compounds as AChE and BACE-1 inhibitors. ACS Med Chem Lett 7:470–475
Cummings JL, Morstorf T, Zhong K (2007) Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res Ther 6:1–7
Delogu GL, Matos MJ, Fanti M, Era B, Medda R, Pieroni E, Fai A, Kumar A, Pintus F (2016) 2-Phenylbenzofuran derivatives as butyrylcholinesterase inhibitors: Synthesis, biological activity and molecular modeling. Bioorg Med Chem Lett 26:2308–2313
Dvir H, Silman I, Harel M, Rosenberry TL, Sussmana JL (2010) Acetylcholinesterase: from 3D structure to function. Chem Biol Interact 187:10–22
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharm 7:88–95
Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelley M, Perry JK (2004) Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem 47:1739–1749
Friesner RA, Murphy RB, Repasky MP, Frye LL, Greenwood JR, Halgren TA, Sanschagrin PC, Mainz DT (2006) Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J Med Chem 49:6177–6196
Gilhus NE, Skeie GO, Romi F, Lazaridis K, Zisimopoulou P, Tzartos S (2016) Myasthenia gravis - autoantibody characteristics and their implications for therapy. Nat Rev Neurol 12(5):259–268
Groner E, Ashani Y, Schorer-Apelbaum D, Sterling J, Herzig Y, Weinstock M (2007) The kinetics of inhibition of human acetylcholinesterase and butyrylcholinesterase by two series of novel carbamates. Mol Pharmacol 71(6):1610–1617
Halgren TA, Murphy RB, Friesner RA, Beard HS, Frye LL, Pollard WT, Banks JL (2004) Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J Med Chem 47:1750–1759
Huang L, Su T, Shan W, Luo Z, Sun Y, He F, Li X (2012) Inhibition of cholinesterase activity and amyloid aggregation by berberine-phenyl-benzoheterocyclic and tacrine-phenyl-benzoheterocyclic hybrids. Bioorg Med Chem 20:3038–3048
Jiang H, Wang X, Huang L, Luo Z, Su T, Ding K, Lia X (2011) Benzenediol-berberine hybrids: Multifunctional agents for Alzheimer’s disease. Bioorg Med Chem 19:7228–7235
Kneza D, Brusa B, Coquelleb N, Sosiča I, Šinka R, Brazzolottoe X, Mravljaka J, Colletierb JP, Gobeca S (2015) Structure-based development of nitroxoline derivatives as potential multifunctional anti-Alzheimer agents. Bioorg Med Chem 23:4442–4452
Leon J, Marco-Contelles J (2011) A step further towards multitarget drugs for Alzheimer and neuronal vascular diseases: targeting the cholinergic system, amyloid-β aggregation and Ca2+ dyshomeostatis. J Curr Med Chem 18:552
Li B, Duysen EG, Carlson M, Lockridge O (2008) The butyrylcholinesterase knockout mouse as a model for human butyrylcholinesterase deficiency. J Pharmacol Exp Ther 324:1146–1154
Mehta M, Adem A, Sabbagh M (2012) New acetylcholinesterase inhibitors for Alzheimer’s disease. Int J Alzheimers Dis. doi:10.1155/2012/728983
Mesulam MM, Guillozet A, Shaw P, Levey A, Duysen E, Lockridge O (2002) Acetylcholinesterase knockouts establish central cholinergic pathways and can use butyrylcholinesterase to hydrolyze acetylcholine. Neuroscience 110:627–639
Nachon F, Carletti E, Ronco C, Trovaslet M, Nicolet Y, Jean L, Renard PY (2013) Crystal structures of human cholinesterases in complex with huprine W and tacrine: elements of specificity for anti-Alzheimer’s drugs targeting acetyl- and butyryl-cholinesterase. Biochem J 453:393–399
Nagle P, Pawar Y, Sonawane A, Bhosale S, More D (2014) Docking simulation, synthesis and biological evaluation of novel pyridazinone containing thymol as potential antimicrobial agents. Med Chem Res 23:918–926
Nepovimova E, Uliassi E, Korabecny J, Peña-Altamira LE, Samez S, Pesaresi A, Garcia GE, Bartolini M, Andrisano V, Bergamini C, Fato R, Lamba D, Roberti M, Kuca K, Monti B, Bolognesi ML (2014) Multitarget drug design strategy: quinone–tacrine hybrids designed To block Amyloid-β aggregation and to exert anticholinesterase and antioxidant effects. J Med Chem 57(20):8576–8589
Nicolet Y, Lockridge O, Masson P, Fontecilla-Camps JC, Nachon F (2003) Crystal structure of human butyrylcholinesterase and of its complexes with substrate and products. J Biol Chem 278:41141–41147
Orhan I, Aslan S, Kartal M, Şener B, Başer KHC (2008) Inhibitory effect of Turkish Rosmarinus officinalis L. on acetylcholinesterase and butyrylcholinesterase enzymes. Food Chem 108:663–668
Otto R, Penzis R, Gaube F, Winckler T, Appenroth D, Fleck C, Treankle C, Lehmann J, Enzensperger C (2014) Enzensperger, Beta and gamma carboline derivatives as potential anti-Alzheimer agents: a comparison. Eur J Med Chem 87:63–70
Önkol T, Gökçe M, Orhan İ, Kaynak F (2013) Design, synthesis and evaluation of some novel 3(2H)-pyridazinone-2-yl acetohydrazides as acetylcholinesterase and butyrylcholnesterase inhibitors. Org Commun 6:55–67
Özçelik AB, Gökçe M, Orhan İ, Kaynak F, Şahin MF (2010) Synthesis and antimicrobial, acetylcholinesterase and butyrylcholinesterase inhibitory avtivities of novel ester and hydrazide derivatives of 3(2H)-pyridazinone. Arzneim Forsch 60(7):452–458
Rathish IG, Javed K, Ahmad S, Bano S, Alam MS, Akhter M, Pillai KK, Ovais S, Samim M (2012) Synthesis and evaluation of anticancer activity of some novel 6-aryl-2-(p-sulfamylphenyl)-pyridazin-3(2H)-ones. Eur J Med Chem 49:304–309
Romero A, Cacabelos R, Oset-Gasque MJ, Samadi A, Marco-Contelles J (2013) Novel tacrine-related drugs as potential candidates for the treatment of Alzheimer’s disease. Bioorg Med Chem Lett 23(7):1916–1922
Saeed A, Mahesara PA, Zaibb S, Khanb MS, Matinc A, Shahidd M, Iqbalb J (2014) Synthesis, cytotoxicity and molecular modelling studies of new phenylcinnamide derivatives as potent inhibitors of cholinesterases. Eur J Med Chem 78:43–53
Saeed A, Zaib S, Ashraf S, Iftikhar J, Muddassar M, Zhang KYJ, Iqbal J (2015) Synthesis, cholinesterase inhibition and molecular modelling studies of coumarin linked thiourea derivatives. Bioorg Chem 63:58–63
Samadi A, De los Ríos C, Bolea I, Chioua M, Iriepa I, Moraleda I, Bartolini M, Andrisano V, Galvez E, Valderas C, Unzeta M, Marco-Contelles J (2012) Multipotent MAO and cholinesterase inhibitors for the treatment of Alzheimer’s disease: synthesis, pharmacological analysis and molecular modeling of heterocyclic substituted alkyl and cycloalkyl propargyl amine. Eur J Med Chem 52:251–262
Shah MS, Khan SU, Ejaz SA, Afridi S, Rizvi SUF, Najam-ul-Haq M, Iqbal J (2016) Cholinesterases inhibition and molecular modeling studies of piperidyl-thienyl and 2-pyrazoline derivatives of chalcones. Biochem Biophys Res Commun xxx:1–10
Siddiqui AA, Mishra R, Shaharyar M (2010) Synthesis, characterization and antihypertensive activity of pyridazinone derivatives. Eur J Med Chem 45:2283–2290
Siddiqui AA, Mishra R, Shaharyar M, Husain A, Rashid M, Pal P (2011) Triazole incorporated pyridazinones as a new class of antihypertensive agents: Design, synthesis and in vivo screening. Bioorg Med Chem Lett 21:1023–1026
Simoni E, Daniele S, Bottegoni G, Pizzirani D, Trincavelli ML, Goldoni L, Tarozzo G, Reggiani A, Martini C, Piomelli D, Melchiorre C, Rosini M, Cavalli A (2012) Combining Galantamine and Memantine in Multitargeted, New Chemical Entities Potentially Useful in Alzheimer’s Disease. J Med Chem 55(22):9708–9721
Strelnik AD, Petukhov AS, Zueva AV, Zobov VV, Petrov KA, Nikolsky EE, Balakin KV, Bachurin SO, ShtyrlinYG (2016) Novel potent pyridoxine-based inhibitors of AChE and BChE, structural analogs of pyridostigmine, with improved in vivo safety profile. Bioorg Med Chem Lett 26:4092–4094
Şahin MF, Badıçoglu B, Gökçe M, Küpeli E, Yeşilada E (2004) Synthesis and analgesic and antiinflammatory activity of methyl [6-substitue-3(2H)- pyridazinone-2-yl]acetate derivatives. Arch Pharm Pharm Med 33:445–452
Tampi RR, Tampi DJ, Ghori AK (2016) Acetylcholinesterase inhibitors for delirium in older adults. Am J Alzheimer Dis 31(4):305–310
Tougu V (2001) Acetylcholinesterase: mechanism of catalysis and inhibition. Curr Med Chem Cent Nerv Syst Agents 1:155–170
Utku S, Gökçe M, Aslan G, Bayram G, Ülger M, Emekdaş G, Şahin MF (2011a) Synthesis and in vitro antimycobacterial activities of novel 6-substituted-3(2H)-pyridazinone-2-acetyl-2-(substituted/nonsubstituted acetophenone)hydrazone. Turk J Chem 35:331–339
Utku S, Gökçe M, Orhan İ, Şahin MF (2011b) Synthesis of novel 6-substituted-3(2H)-pyridazinone-2-acetyl-2-(substituted/-nonsubstituted benzal)hydrazone derivatives and acetylcholinesterase and butyrylcholinesterase inhibitory activities in vitro. Arzneim Forsch 61:1–7
Williams P, Sorribas A, Howes M-JR (2011) Natural products as a source of Alzheimer’s drug leads. Nat Prod Rep 28:48–77
Xing W, Fu Y, Shi Z, Lu D, Zhang H, Hu Y (2013) Discovery of novel 2,6-disubstituted pyridazinone derivatives as acetylcholinesterase inhibitors. Eur J Med Chem 63:95–103
Yamali C, Ozan GH, Kahya B, Çobanoğlu S, Şüküroğlu MK, Doğruer DS (2015) Synthesis of some 3(2H)-pyridazinone and 1(2H) -phthalazinone derivatives incorporating aminothiazole moiety and investigation of their antioxidant, acetylcholinesterase, and butyrylcholinesterase inhibitory activities. Med Chem Res 24:1210–1217
Zha X, Lamba D, Zhang L, Lou Y, Xu C, Kang D, Chen L, Xu Y, Zhang L, De Simone A, Samez S, Pesaresi A, Stojan J, Lopez MG, Egea J, Andrisino V, Bartolini M (2016) Novel tacrine-benzofuran hybrids as potent multitarget-directed ligands for the threatment of Alzheimer’s disease: design, synthesis, biological evaluation and X-ray crystallography. J Med Chem 59:114–131
Zhou Y, Wang S, Zhang Y (2010) Catalytic reaction mechanism of acetylcholinesterase determined by born-oppenheimer AB initio QM/MM molecular dynamics simulations. J Phys Chem B 114:8817–8825
Acknowledgements
This study was funded by the Research Foundation of İnönü University (2013/94).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Özdemir, Z., Yılmaz, H., Sarı, S. et al. Design, synthesis, and molecular modeling of new 3(2H)-pyridazinone derivatives as acetylcholinesterase/butyrylcholinesterase inhibitors. Med Chem Res 26, 2293–2308 (2017). https://doi.org/10.1007/s00044-017-1930-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-017-1930-x