Abstract
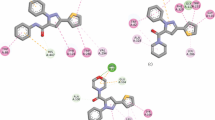
A novel hybrid series of umbellipherone and benzyl amine scaffolds, linked via triazole ring, was synthesized and evaluated as both acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) inhibitors. Most of the synthesized compounds showed moderate to high activities by using Ellman’s modified assay. Among the target compounds, 6e bearing 3-methoxy substituent on benzyl moiety was the most active one (AChE and BuChE IC50 = 3.4 and 1.1 μM, respectively). Finally, binding modes of the target compound was studied using molecular docking stimulations. The neuroprotectivity evaluation exhibited that this compound efficiently protected PC12 neurons against H2O2-induced cell death.




Similar content being viewed by others
References
Ali MY, Jannat S, Jung HA, Choi RJ, Roy A, Choi JS (2016) Anti-Alzheimer’s disease potential of coumarins from Angelica decursiva and Artemisia capillaris and structure-activity analysis. Asian Pac J Trop Med 9:103–111
Alipour M, Khoobi M, Moradi A, Nadri H, Moghadam FH, Emami S, Hasanpour Z, Foroumadi A, Shafiee A (2014) Synthesis and anti-cholinesterase activity of new 7-hydroxycoumarin derivatives. Eur J Med Chem 82:536–544
Anand P, Singh B, Singh N (2012) A review on coumarins as acetylcholinesterase inhibitors for Alzheimer’s disease. Bioorg Med Chem 20:1175–1180
Ávila J, Lim F, Moreno F, Belmonte C, Cuello AC (2002) Tau function and dysfunction in neurons. Mol Neurobiol 25:213–231
Bagheri SM, Khoobi M, Nadri H, Moradi A, Emami S, Jalili‐Baleh L, Jafarpour F, Moghadam FH, Foroumadi A, Shafiee A (2015) Synthesis and structure-activity relationship study of tacrine-based pyrano [2,3-c] pyrazoles targeting AChE/BuChE and 15-LOX. Chem Biol Drug Des 86:1215–1220
Blokland A (1995) Acetylcholine: a neurotransmitter for learning and memory. Brain Res Rev 21:285–300
Bukar Maina M, Al-Hilaly YK, Serpell LC (2016) Nuclear tau and its potential role in Alzheimer’s disease. Biomolecules 6:9–28
Burke A, Hall G, Yaarl R, Fleisher A, Dougherty J, Young J, Brand H, Tariot P (2015) Pocket Reference to Alzheimer’s Disease Management. Springer Healthcare Limited, New York, NY, p 35–40
Cecilia Rodrigues Simoes M, Pereira Dias Viegas F, Soares Moreira M, de Freitas Silva M, Maximo Riquiel M, Mattos da Rosa P, Rosa Castelli M, Henrique dos Santos M, Gomes Soares M, Viegas C (2014) Donepezil: an important prototype to the design of new drug candidates for Alzheimer’s disease. Mini Rev Med Chem 14:2–19
Correia SC, Resende R, Moreira PI, Pereira CM (2015) Alzheimer’s disease-related misfolded proteins and dysfunctional organelles on autophagy menu. DNA Cell Biol 34:261–273
De Ferrari GV, Canales MA, Shin I, Weiner LM, Silman I, Inestrosa NC (2001) A structural motif of acetylcholinesterase that promotes amyloid β-peptide fibril formation. Biochemistry 40:10447–10457
Dougherty DA, Stauffer DA (1990) Acetylcholine binding by a synthetic receptor: implications for biological recognition. Science 250:1558–1561
Duan S, Guan X, Lin R, Liu X, Yan Y, Lin R, Zhang T, Chen X, Huang J, Sun X, Li Qn (2015) Silibinin inhibits acetylcholinesterase activity and amyloid β peptide aggregation: a dual-target drug for the treatment of Alzheimer’s disease. Neurobiol Aging 36:1792–1807
Dvir H, Silman I, Harel M, Rosenberry TL, Sussman JL (2010) Acetylcholinesterase: from 3D structure to function. Chem Biol Interact 187:10–22
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88IN191. -9095
Gendron TF, Petrucelli L (2009) The role of tau in neurodegeneration. Mol Neurodegener 4:13
Greig NH, Utsuki T, Yu QS, Zhu X, Holloway HW, Perry T, Lee B, Ingram DK, Lahiri DK (2001) A new therapeutic target in Alzheimer’s disease treatment: attention to butyrylcholinesterase. Curr Med Res Opin 17:159–165
Greig NH, Lahiri DK, Sambamurti K (2002) Butyrylcholinesterase: an important new target in Alzheimer’s disease therapy. Int Psychogeriatr 14:77–91
Himo F, Lovell T, Hilgraf R, Rostovtsev VV, Noodleman L, Sharpless KB, Fokin VV (2005) Copper (I)-catalyzed synthesis of azoles. DFT study predicts unprecedented reactivity and intermediates.J Am Chem Soc 127(1):210–216
Mao F, Huang L, Luo Z, Liu A, Lu C, Xie Z, Li X (2012) O-Hydroxyl-or o-amino benzylamine-tacrine hybrids: multifunctional biometals chelators, antioxidants, and inhibitors of cholinesterase activity and amyloid-β aggregation. Bioorg Med Chem 20:5884–5892
O’Boyle NM1, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR (2011) Open Babel: An open chemical toolbox. J. Cheminform 3:33–36
Palmer AM (2011) Neuroprotective therapeutics for Alzheimer’s disease: progress and prospects. Trends Pharmacol Sci 32:141–147
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612
Rhein V, Song X, Wiesner A, Ittner LM, Baysang G, Meier F, Ozmen L, Bluethmann H, Dröse S, Brandt U, Savaskan E (2009) Amyloid-β and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer’s disease mice. Proc Natl Acad Sci 106:20057–20062
Sanner MF (1999) Python: a programming language for software integration and development. J Mol Graph Model 17:57–61
Selkoe DJ (2001) Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev 81:741–766
Selkoe DJ (2004) Cell biology of protein misfolding: the examples of Alzheimer’s and Parkinson’s diseases. Nat Cell Biol 6:1054–1061
Subramaniam SR, Ellis EM (2013) Neuroprotective effects of umbelliferone and esculetin in a mouse model of Parkinson’s disease. J Neurosci Res 91:453–461
Torres FC, Gonçalves GA, Vanzolini KL, Merlo AA, Gauer B, Holzschuh M, Andrade S, Piedade M, Garcia SC, Carvalho I, Poser GL (2016) Combining the pharmacophore features of coumarins and 1, 4-substituted 1, 2, 3-triazoles to design new acetylcholinesterase inhibitors: Fast and easy generation of 4-methylcoumarins/1, 2, 3-triazoles conjugates via click chemistry. J Braz Chem Soc 27:1541–1550
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31:455–461
Venugopala KN, Rashmi V, Odhav B (2013) Review on natural coumarin lead compounds for their pharmacological activity. BioMed Res Int 2013:963248
Zheng H, Koo EH (2006) The amyloid precursor protein: beyond amyloid. Mol Neurodegener 1:5–17
Acknowledgements
This work was supported by grants from the Research Council of Tehran University of Medical Sciences and Iran National Science Foundation (INSF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Moradi, A., Faraji, L., Nadri, H. et al. Synthesis, docking study, and biological evaluation of novel umbellipherone/hymecromone derivatives as acetylcholinesterase/butyrylcholinesterase inhibitors. Med Chem Res 27, 1741–1747 (2018). https://doi.org/10.1007/s00044-018-2187-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-018-2187-8