Abstract
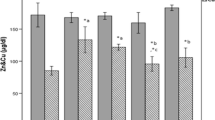
Diabetes is associated with increased oxidative stress and impaired antioxidant defenses. Thioredoxin-1 (TRX-1) is a cytosolic thiol antioxidant and redox-active protein which plays a vital role in the maintenance of reduced intracellular redox state. In this study, the authors examined whether 4-week treatments with sodium selenate and doxycycline—a metalloproteinase-2 inhibitor which also has antioxidant-like effects—offset perturbations in oxidative stress and antioxidant protection in rat liver and skeletal muscle in streptozotocin-induced diabetes (SID) model. Experimental diabetes decreased TRX-1 levels in skeletal muscle and liver. On the other hand, SID increased oxidative stress marker protein carbonyl levels and decreased oxygen radical absorbance capacity (ORAC), an indicator of antioxidant capacity, in liver. A 4-week treatment of sodium selenate to diabetic rats decreased blood glucose levels moderately, while doxycycline treatment caused a reduction in weight loss of diabetic rats. Both doxycycline and sodium selenate prevented diabetes-induced decrease of TRX-1 levels in skeletal muscle, whereas only doxyxycline was effectively preventing diabetes-induced decrease of TRX-1 in liver. Furthermore, both treatments prevented diabetes-induced altered levels of protein carbonyls and ORAC in liver, and restored free and total protein thiol levels in both skeletal muscle and liver. In conclusion, the data of this study provides further evidence that sodium selenate and doxycycline treatments may control oxidative stress and improve antioxidant defense in diabetes.



Similar content being viewed by others
References
Nishinaka Y, Nakamura H, Masutani H, Yodoi J (2001) Redox control of cellular function by thioredoxin; a new therapeutic direction in host defence. Arch Immunol Ther Exp (Warsz) 49:285–292
Atalay M, Laaksonen DE, Niskanen L, Uusitupa M, Hänninen O, Sen CK (1997) Altered antioxidant enzyme defences in insulin-dependent diabetic men with increased resting and exercise-induced oxidative stres. Acta Physiol Scand 161:195–201
Atalay M, Laaksonen DE (2002) Diabetes, oxidative stress and physical exercise. J Sport Sci Med 1:1–14
Jones DP (2008) Radical-free biology of oxidative stress. Am J Physiol Cell Physiol 295:C849–C868
Cantin AM, Hubbard RC, Crystal RG (1989) Glutathione deficiency in the epithelial lining fluid of the lower respiratory tract in idiopathic pulmonary fibrosis. Am Rev Respir Dis 139:370–372
Cheung PY, Sawicki G, Wozniak M, Wang W, Radomski MW, Schulz R (2000) Matrix metalloproteinase-2 contributes to ischemia-reperfusion injury in the heart. Circulation 101:1833–1839
Sawicki G, Leon H, Sawicka J, Sariahmetoglu M, Schulze CJ, Scott PG, Szczesna-Cordary D, Schulz R (2005) Degradation of myosin light chain in isolated rat hearts subjected to ischemia-reperfusion injury: a new intracellular target for matrix metalloproteinase-2. Circulation 112:544–552
Lemaitre V, D’armiento J (2006) Matrix metalloproteinases in development and disease. Birth Defects Res C 78:1–10
Arnér ES, Holmgren A (2006) The thioredoxin system in cancer. Semin Cancer Biol 16:420–426
Berggren M, Gallegos A, Gasdaska J, Powis G (1997) Cellular thioredoxin reductase activity is regulated by selenium. Anticancer Res 17:3377–3380
Berggren MM, Mangin JF, Gasdaka JR, Powis G (1999) Effect of selenium on rat thioredoxin reductase activity: increase by supranutritional selenium and decrease by selenium deficiency. Biochem Pharmacol 57:187–193
Yamawaki H, Berk BC (2005) Thioredoxin: a multifunctional antioxidant enzyme in kidney, heart and vessels. Curr Opin Nephrol Hypertens 14:149–153
Kakisaka Y, Nakashima T, Sumida Y, Yoh T, Nakamura H, Yodoi J, Senmaru H (2002) Elevation of serum thioredoxin levels in patients with type 2 diabetes. Hom Metab Res 34:160–164
Miyamoto S, Kawano H, Hokamaki J, Soejima H, Kojima S, Kudoh T, Nagayoshi Y, Sugiyama S, Sakamoto T, Yoshimura M, Nakamura H, Yodoi J, Ogawa H (2005) Increased plasma levels of thioredoxin in patients with glucose intolerance. Intern Med 44:1127–1132
Hokamaki J, Kawano H, Soejima H, Miyamoto S, Kajiwara I, Kojima S, Sakamoto T, Sugiyama S, Yoshimura M, Nakamura H, Yodoi J, Ogawa H (2005) Plasma thioredoxin levels in patients with unstable angina. Int J Cardiol 99:225–231
Ayaz M, Ozdemir S, Ugur M, Vassort G, Turan B (2004) Effects of selenium on altered mechanical and electrical cardiac activities of diabetic rat. Arch Biochem Biophys 426:83–90
Can B, Ulusu NN, Kilinç K, Leyla Acan N, Saran Y, Turan B (2005) Selenium treatment protects diabetes-induced biochemical and ultrastructural alterations in liver tissue. Biol Trace Elem Res 105:135–150
Berg EA, Wu JY, Campbell L, Kagey M, Stapleton SR (1995) Insulin-like effects of vanadate and selenate on the expression of glucose-6-phosphate dehydrogenase and fatty acid synthase in diabetic rats. Biochimie 77:919–924
Yaras N, Sariahmetoglu M, Bilginoglu A, Aydemir-Koksoy A, Onay-Besikci A, Turan B, Schulz R (2008) Protective action of doxycycline against diabetic cardiomyopathy in rats. Br J Pharmacol 155:1174–1184
Golub LM, Ramamurthy N, McNamara T, Greenwald R, Rifkin B (1991) Tetracyclines inhibit connective tissue breakdown: new therapeutic implications for an old family of drugs. Crit Rev Oral Biol Med 2:297–322
Golub LM, Lee HM, Ryan ME, Giannobile WV, Payne J, Sorsa T (1998) Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv Dent Res 12:12–26
Yeh YC, Lai HC, Ting CT, Lee WL, Wang LC, Wang KY, Lai HC, Liu TJ (2007) Protection by doxycycline against doxorubicin-induced oxidative stress and apoptosis in mouse testes. Biochem Pharmacol 74:969–980
Soory M (2008) A role for non-antimicrobial actions of tetracyclines in combating oxidative stress in periodontal and metabolic diseases: a literature review. Open Dent J 2:5–12
Lappalainen Z, Lappalainen J, Oksala NKJ, Laaksonen DE, Khanna S, Sen CK, Atalay M (2009) Diabetes impairs exercise training-associated thioredoxin response and glutathione status in rat brain. J Appl Physiol 106:461–467
Prior RL, Hoang H, Gu L, Wu X, Bacchiocca M, Howard L, Hampsch-Woodill M, Huang D, Ou B, Jacob R (2003) Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORAC(FL))) of plasma and other biological and food samples. J Agric Food Chem 51:3273–3279
Kinnunen S, Hyyppä S, Lehmuskero A, Oksala N, Mäenpää P, Hänninen O, Atalay M (2005) Oxygen radical absorbance capacity (ORAC) and exercise-induced oxidative stress in trotters. Eur J Appl Physiol 95:1–7
Oksala NK, Paimela H, Alhava E, Atalay M (2007) Heat shock preconditioning induces protein carbonylation and alters antioxidant protection in superficially injured guinea pig gastric mucosa in vitro. Dig Dis Sci 52:1897–1905
Turan B, Desilets M, Acan LN, Hotomaroglu O, Vannier C, Vassort G (1996) Oxidative effects of selenite on rat ventricular contractility and Ca movements. Cardiovasc Res 32:351–361
Jotty K, Ojeda ML, Nogales F, Rubio JM, Murillo ML, Carreras O (2009) Selenium tissue distribution changes after ethanol exposure during gestation and lactation: selenite as a therapy. Food Chem Toxicol 47(10):2484–2489
Gan L, Liu Q, Xu HB, Zhu YS, Yang XL (2002) Effects of selenium overexposure on glutathione peroxidase and thioredoxin reductase gene expressions and activities. Biol Trace Elem Res 89(2):165–175
Cao G, Shukitt-Hale B, Bickford PC, Joseph JA, McEwen J, Prior RL (1999) Hyperoxia-induced changes in antioxidant capacity and the effect of dietary antioxidants. J Appl Physiol 86:1817–1822
Shacter E (2000) Quantification and significance of protein oxidation samples. Drug Metab Rev 32:307–326
Stadtman ER, Levine RL (2000) Protein oxidation. Ann N Y Acad Sci 899:191–208
Telci A, Cakatay U, Salman S, Satman I, Sivas A (2000) Oxidative protein damage in early stage type 1 diabetic patients. Diabetes Res Clin Pract 50:213–223
Bonnefont-Rousselot D, Bastard JP, Jaudon MC, Delattre J (2000) Consequences of the diabetic status on the oxidant/antioxidant balance. Diabetes Metab 26:163–176
Li X, Xu Z, Li S, Rozanski GJ (2005) Redox regulation of I to remodeling in diabetic rat heart. Am J Physiol Heart Circ Physiol 288:H1417–H1424
Kiersztan A, Baranska A, Hapka M, Lebiedzinska M, Winiarska K, Dudziak M, Bryla J (2009) Differential action of methyselenocysteine in control and alloxan-diabetic rabbits. Chem Biol Interact 27:161–171
Hawkes WC, Alkan Z (2010) Regulation of redox signaling by selenoproteins. Biol Trace Elem Res 134:235–251
Ulusu NN, Turan B (2005) Beneficial effects of selenium on some enzymes of diabetic rat heart. Biol Trace Elem Res 103:207–216
Tuncay E, Seymen AA, Tanriverdi E, Yaras N, Tandogan B, Ulusu NN, Turan B (2007) Gender related differential effects of Omega-3E treatment on diabetes-induced left ventricular dysfunction. Mol Cell Biochem 304:255–263
Ayaz M, Ozdemir S, Yaras N, Vassort G, Turan B (2005) Selenium-induced alterations in ionic currents of rat cardiomyocytes. Biochem Biophys Res Commun 327:163–173; Erratum in: Biochem Biophys Res Commun 329:418
Selenius M, Fernandes AP, Brodin O, Björnstedt M, Rundlöf AK (2008) Treatment of lung cancer cells with cytotoxic levels of sodium selenite: effects on the thioredoxin system. Biochem Pharmacol 75:2092–2099
Ramamurthy NS, Vernillo AT, Greenwald RA, Lee HM, Sorsa T, Golub LM, Rifkin BR (1993) Reactive oxygen species activate and tetracyclines inhibit rat osteoblast collagenase. J Bone Miner Res 8:1247–1253
Ryan ME, Ramamurthy NS, Golub LM (1998) Tetracyclines inhibit protein glycation in experimental diabetes. Adv Dent Res 12:152–158
Acknowledgments
This study has been supported by grants from the Scientific and Technological Research Council of Turkey (TUBITAK)-SBAG-107S427 and TUBITAK-SBAG-107S304, and the European Cooperation in Science and Technology (COST) action BM0602 to BT, from the Finnish Ministry of Education and COST actions B35 and BM0602 to MA. AB is supported by the ERASMUS program.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Atalay, M., Bilginoglu, A., Kokkola, T. et al. Treatments with sodium selenate or doxycycline offset diabetes-induced perturbations of thioredoxin-1 levels and antioxidant capacity. Mol Cell Biochem 351, 125–131 (2011). https://doi.org/10.1007/s11010-011-0719-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-011-0719-3