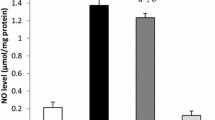
Abstract
Increased oxidative stress and impaired antioxidant defense mechanisms are believed to be the important factors contributing to the pathogenesis and progression of diabetes mellitus. In this study, we have reported the effects of the streptozotocin-induced diabetes on the gene expression and the activities of two antioxidant enzymes, manganese superoxide dismutase (MnSOD) and glutathione peroxidase (GPx). We also studied the effects of two antioxidants, vitamin C and DL-α-lipoic acid (LA), on the system. Our results showed no significant change in both enzymes activities in diabetic animals compared to controls. Similarly, mRNA and protein profiles of MnSOD showed no change. Though the mRNA expression of GPx did not show any change, Western-blot analysis results demonstrated that protein expression is increased. LA, which is a water- and lipid-soluble antioxidant, decreased the protein expression of MnSOD, though mRNA levels and activities remained unchanged. LA treatment increased the GPx activities in diabetic tissues, significantly, and RT-PCR and Western-blot analysis results demonstrated that this increase in activity is not regulated at the gene level, as both mRNA and protein levels did not change. Supplementing the animals with vitamin C, a powerful water-soluble antioxidant, increased the mRNA expression of MnSOD, though the protein expression and the activity did not change statistically. On the other hand GPx activity increased significantly through post-translational modifications, as both mRNA and protein expressions did not change. These results together with our previous findings about the gene expressions of catalase and Cu–Zn SOD indicate the presence of very intricate control mechanisms regulating the activities of antioxidant enzymes in order to prevent the damaging effects of oxidative stress.






Similar content being viewed by others
References
Hunt JV, Smith CC, Wolff SP (1990) Autoxidative glycosylation and possible involvement of peroxides and free radicals in LDL modification by glucose. Diabetes 39:1420–1424. doi:10.2337/diabetes.39.11.1420
Wolff SP, Dean RT (1987) Glucose autoxidation and protein modification. The potential role of autoxidative glycosylation in diabetes. Biochem J 245:243–250
Chung SS, Ho EC, Lam KS et al (2003) Contribution of polyol pathway to diabetes-induced oxidative stress. J Am Soc Nephrol 14:233–236. doi:10.1097/01.ASN.0000077408.15865.06
Brownlee M (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414:813–820. doi:10.1038/414813a
Chance B, Sies H, Boveris A (1979) Hydroperoxide metabolism in mammalian organs. Physiol Rev 59:527–605
Imai H, Narashima K, Arai M et al (1998) Suppression of leukotriene formation in RBL-2H3 cells that overexpressed phospholipid hydroperoxide glutathione peroxidase. J Biol Chem 273:1990–1997. doi:10.1074/jbc.273.4.1990
Mates JM, Pérez-Gómez C, Castroa IND (1999) Antioxidant enzymes and human diseases. Clin Biochem 32(8):595–603. doi:10.1016/S0009-9120(99)00075-2
Maritim AC, Sanders RA, Watkins JB (2003) Effects of alpha-lipoic acid on biomarkers of oxidative stress in streptozotocin-induced diabetic rats. J Nutr Biochem 14(5):288–294. doi:10.1016/S0955-2863(03)00036-6
Padayatty SJ, Daruwala R, Wang YY, et al (2002) Vitamin C: from molecular actions to optimum intake. In: Cadenas E, Packer L (eds) Handbook of antioxidants (oxidative stress and disease) (Chap. 7), 2nd edn. CRC Press, Boca Raton
Sadi G, Yilmaz O, Güray T (2008) Effect of vitamin C and lipoic acid on streptozotocin-induced diabetes gene expression: mRNA and protein expressions of Cu–Zn SOD and catalase. Mol Cell Biochem 309(1–2):109–116. doi:10.1007/s11010-007-9648-6
Giles AR (1987) Guidelines for the use of animals in biomedical research. Thromb Haemost 58:1078–1084
Lowry OH, Rosebrough NJ, Farr AL et al (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Marklund SL, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474. doi:10.1111/j.1432-1033.1974.tb03714.x
Paglia ED, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocytes glutathione peroxides. J Lab Clin Med 70:158–169
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76(9):4350–4354. doi:10.1073/pnas.76.9.4350
Rasband WS (2008) ImageJ, US National Institutes of Health, Bethesda, Maryland, USA, http://rsb.info.nih.gov/ij/
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159. doi:10.1016/0003-2697(87)90021-2
Yu BP (1994) Cellular defence against damage from reactive oxygen species. Physiol Rev 74:139–162
Kono Y, Fridovich I (1982) Superoxide radical inhibits catalase. J Biol Chem 257(10):5751–5754
Sen CK, Packer L (1996) Antioxidant and redox regulation of gene transcription. FASEB J 10(7):709–720
Stralin P, Marklund SL (1994) Effects of oxidative stress on expression of extracellular superoxide dismutase, CuZn-superoxide dismutase and Mn-superoxide dismutase in human dermal fibroblasts. Biochem J 298:347–352
Liu RG, Buettner GR, Oberley LW (2000) Oxygen free radicals mediate the induction of manganese superoxide dismutase gene expression by TNF-alpha. Free Radic Biol Med 28(8):1197–1205. doi:10.1016/S0891-5849(00)00237-9
Dobashi K, Ghosh B, Orak JK et al (2000) Kidney ischemia-reperfusion: modulation of antioxidant defenses. Mol Cell Biochem 205:1–11. doi:10.1023/A:1007047505107
Cobbs CS, Levi DS, Aldape K et al (1996) Manganese superoxide dismutase expression in human central nervous system tumors. Cancer Res 56:3192–3195
Taniguchi N (1992) Clinical significances of superoxide dismutases: changes in aging, diabetes, ischemia, and cancer. Adv Clin Chem 29:1–59. doi:10.1016/S0065-2423(08)60221-8
Macmillan-Crow LA, Cruthirds DL (2001) Manganese superoxide dismutase in disease. Free Radic Res 34(4):325–336. doi:10.1080/10715760100300281
Sindhu RK, Koo JR, Roberts CK et al (2004) Dysregulation of hepatic superoxide dismutase, catalase and glutathione peroxidase in diabetes: response to insulin and antioxidant therapies. Clin Exp Hyp 26(1):43–53. doi:10.1081/CEH-120027330
Bhor VM, Raghuram N, Sivakami S (2004) Oxidative damage and altered antioxidant enzyme activities in the small intestine of streptozotocin-induced diabetic rats. Int J Biochem Cell Biol 36:89–97. doi:10.1016/S1357-2725(03)00142-0
Limaye PV, Raghuram N, Sivakami S (2003) Oxidative stress and gene expression of antioxidant enzymes in the renal cortex of streptozotocin-induced diabetic rats. Mol Cell Biochem 243:147–152. doi:10.1023/A:1021620414979
Kakkar R, Mantha SV, Radhi J et al (1998) Increased oxidative stress in rat liver and pancreas during progression of streptozotocin-induced diabetes. Clin Sci 94(6):623–632
Ceriello A, Morocutti A, Mercuri F et al (2000) Defective intracellular antioxidant enzyme production in type 1 diabetic patients with nephropathy. Diabetes 49(12):2170–2177. doi:10.2337/diabetes.49.12.2170
Packer L, Kraemer K, Rimbach G (2001) Molecular aspects of lipoic acid in the prevention of diabetic complications. Nutrition 17:888–895. doi:10.1016/S0899-9007(01)00658-X
Dincer Y, Telci A, Kayalı R et al (2002) Effect of α-lipoic acid on lipid peroxidation and antioxidant enzyme activities in diabetic rats. Clin Exp Pharm Phys 29:281–284. doi:10.1046/j.1440-1681.2002.03642.x
Moini H, Packer L, Saris NE (2002) Antioxidant and prooxidant activities of alpha-lipoic acid and dihydrolipoic acid. Toxicol Appl Pharmacol 182(1):84–90. doi:10.1006/taap.2002.9437
Obrosova IG, Fathallah L, Liu E et al (2003) Early oxidative stress in the diabetic kidney: effect of DL-alpha-lipoic acid. Free Radic Biol Med 34(2):186–195. doi:10.1016/S0891-5849(02)01195-4
Halliwell B, Gutteridge JMC (1999) Free radicals in biology and medicine, 3rd edn. Clarendon Press, Oxford
Lutsenko EA, Carcamo JM, Golde DW (2002) Vitamin C prevents DNA mutation induced by oxidative stress. J Biol Chem 277(19):16895–16899. doi:10.1074/jbc.M201151200
Garg MC, Bansal DD (2000) Protective antioxidant effect of vitamins C and E in streptozotocin induced diabetic rats. Indian J Exp Biol 38(2):101–104
Knirsch L, Clerch LB (2001) Tyrosine phosphorylation regulates manganese superoxide dismutase (MnSOD) RNA-binding protein activity and MnSOD protein expression. Biochem 40(26):7890–7895. doi:10.1021/bi010197n
Cao C, Leng YM, Huang W et al (2003) Glutathione peroxidase 1 is regulated by the c-Abl and Arg tyrosine kinases. J Biol Chem 278(41):39609–39614. doi:10.1074/jbc.M305770200
Acknowledgements
The financial support provided by grants from Middle East Technical University (BAP-08-11-DPT2002K120510-TB3) and TUBITAK (108T295) are gratefully acknowledged. We also thank Assoc.Prof.Dr.Ökkeş Yılmaz for providing the animals.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sadi, G., Güray, T. Gene expressions of Mn-SOD and GPx-1 in streptozotocin-induced diabetes: effect of antioxidants. Mol Cell Biochem 327, 127–134 (2009). https://doi.org/10.1007/s11010-009-0050-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-009-0050-4