Abstract
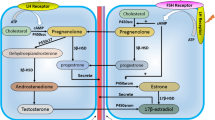
OAS1 belongs to a protein family of interferon-induced enzymes characterized by their ability to catalyze the synthesis of 2′–5′-linked oligomers of adenosine from ATP (2-5A). 2-5A bind to the latent Ribonuclease L (RNase L), which subsequently dimerizes into the active form, acquiring the capacity of cleaving cellular and viral mRNA. Several studies indicate that OAS1 is an important inducer of apoptosis in human cancer cells and that it may be regulated by 17β-estradiol (E2). The aim of this study was to characterize OAS1 gene expression in rat mammary gland and prostate, and to analyze its regulation by E2 in both tissues. It is demonstrated that OAS1g is the most abundant OAS1 gene expressed in both tissues, and that OAS1 protein is present in the nucleus of rat mammary gland and prostate epithelial cells. In addition, it is shown by Real Time PCR that OAS1g is up-regulated by E2 in rat mammary gland, but down-regulated in prostate, suggesting that the OAS1g gene may be related to estrogen dependent pathways in rat mammary gland and prostate physiology.






Similar content being viewed by others
References
Sarkar SN, Ghosh A, Wang HW et al (1999) The nature of the catalytic domain of 2′–5′-oligoadenylate synthetases. J Biol Chem 274:25535–25542
Rebouillat D, Hovanessian AG (1999) The human 2′,5′-oligoadenylate synthetase family: interferon-induced proteins with unique enzymatic properties. J Interferon Cytokine Res 19:295–308
Dong B, Silverman RH (1995) 2-5A-dependent RNase molecules dimerize during activation by 2-5A. J Biol Chem 270:4133–4137
Castelli JC, Hassel BA, Maran A et al (1998) The role of 2′–5′ oligoadenylate-activated ribonuclease L in apoptosis. Cell Death Differ 5:313–320
Xiang Y, Wang Z, Murakami J et al (2003) Effects of RNase L mutations associated with prostate cancer on apoptosis induced by 2’,5’-oligoadenylates. Cancer Res 63:6795–6801
Malathi K, Paranjape JM, Ganapathi R et al (2004) HPC1/RNASEL mediates apoptosis of prostate cancer cells treated with 2’,5’-oligoadenylates, topoisomerase I inhibitors, and tumor necrosis factor-related apoptosis-inducing ligand. Cancer Res 64:9144–9151
Mullan PB, Hosey AM, Buckley NE et al (2005) The 2,5 oligoadenylate synthetase/RNaseL pathway is a novel effector of BRCA1- and interferon-gamma-mediated apoptosis. Oncogene 24:5492–5501
Viano I, Silvestro L, Giubertoni M et al (1989) Induction of 2′–5′ oligoadenylate synthetase and activation of ribonuclease in tamoxifen treated human breast cancer cell lines. J Biol Regul Homeost Agents 3:167–174
Tsai MH, Cook JA, Chandramouli GV et al (2007) Gene expression profiling of breast, prostate, and glioma cells following single versus fractionated doses of radiation. Cancer Res 67:3845–3852
Benech P, Merlin G, Revel M et al (1985) 3′ end structure of the human (2′–5′) oligo A synthetase gene: prediction of two distinct proteins with cell type-specific expression. Nucleic Acids Res 13:1267–1281
Kakuta S, Shibata S, Iwakura Y (2002) Genomic structure of the mouse 2’,5’-oligoadenylate synthetase gene family. J Interferon Cytokine Res 22:981–993
Mashimo T, Glaser P, Lucas M et al (2003) Structural and functional genomics and evolutionary relationships in the cluster of genes encoding murine 2′,5′-oligoadenylate synthetases. Genomics 82:537–552
Perelygin AA, Scherbik SV, Zhulin IB et al (2002) Positional cloning of the murine flavivirus resistance gene. Proc Natl Acad Sci USA 99:9322–9327
Eskildsen S, Hartmann R, Kjeldgaard NO et al (2002) Gene structure of the murine 2′–5′-oligoadenylate synthetase family. Cell Mol Life Sci 59:1212–1222
Perelygin AA, Zharkikh AA, Scherbik SV et al (2006) The mammalian 2’–5’ oligoadenylate synthetase gene family: evidence for concerted evolution of paralogous Oas1 genes in Rodentia and Artiodactyla. J Mol Evol 63:562–576
Shibata S, Kakuta S, Hamada K et al (2001) Cloning of a novel 2′,5′-oligoadenylate synthetase-like molecule, Oasl5 in mice. Gene 271:261–271
Smekens M, Dumont JE, Degeyter A et al (1986) Effect of estrogen administration on rat liver 2-5A synthetase activity. Biochim Biophys Acta 887:341–344
Silvestro L, Viano I, Giubertoni M et al (1989) Induction of 2′–5′ oligoadenylate synthetase by 17β-oestradiol in a human breast cancer cell line. Pharmacol Res 21:99–100
Zhang Z, Duan L, Du X et al (2008) The proliferative effect of estradiol on human prostate stromal cells is mediated through activation of ERK. Prostate 68:508–516
Un-no T, Hayami S, Nobata S et al (2007) Neonatal exposure to estrogen in the Wistar rat decreases estrogen receptor-β and induces epithelial proliferation of the prostate in the adult. Urol Int 79:345–351
Altiok N, Koyuturk M, Altiok S (2006) JNK pathway regulates estradiol-induced apoptosis in hormone-dependent human breast cancer cells. Breast Cancer Res Treat 105:247–254
Pattarozzi A, Gatti M, Barbieri F et al (2008) 17β-estradiol promotes breast cancer cell proliferation-inducing stromal cell-derived factor-1-mediated epidermal growth factor receptor transactivation: reversal by gefitinib pretreatment. Mol Pharmacol 73:191–202
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Wang F, Samudio I, Safe S (2001) Transcriptional activation of cathepsin D gene expression by 17β-estradiol: mechanism of aryl hydrocarbon receptor-mediated inhibition. Mol Cell Endocrinol 172:91–103
Gasteiger E, Gattiker A, Hoogland C et al (2003) ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res 31:3784–3788
Chebath J, Benech P, Hovanessian A et al (1987) Four different forms of interferon-induced 2′,5′-oligo(A) synthetase identified by immunoblotting in human cells. J Biol Chem 262:3852–3857
Lobanova YS, Scherbakov AM, Shatskaya VA et al (2007) Mechanism of estrogen-induced apoptosis in breast cancer cells: role of the NF-kappaB signaling pathway. Biochemistry (Mosc) 72:320–327
Harkonen PL, Makela SI (2004) Role of estrogens in development of prostate cancer. J Steroid Biochem Mol Biol 92:297–305
Acknowledgments
Maia CJB is recipient of a fellowship from the Portuguese Foundation for Science and Technology-FCT, SFRH/BD/13388/2003. All the experimental work was supported by the FCT plurianual program. We acknowledge Catarina Ferreira for the preparation of tissue sections and Dr Graça Baltazar for counseling in fluorescence microscopy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maia, C.J.B., Socorro, S., Schmitt, F. et al. Characterization of oligoadenylate synthetase-1 expression in rat mammary gland and prostate: effects of 17β-estradiol on the regulation of OAS1g in both tissues. Mol Cell Biochem 314, 113–121 (2008). https://doi.org/10.1007/s11010-008-9771-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-008-9771-z