Abstract
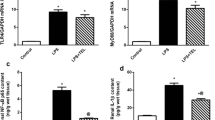
Endotoxins (lipopolysaccharides; LPS) are known to cause multiple organ failure, including renal dysfunction. LPS triggers the synthesis and release of cytokines and the vasodilatör nitric oxide (NO•). A major contributor to the increase in NO• production is LPS-stimulated expression of inducible nitric oxide synthase (iNOS). This occurs in vasculature and most organs including the kidney. During endotoxemia, NO• and superoxide react spontaneously to form the potent and versatile oxidant peroxynitrite (ONOO−) and the formation of 3-nitrotyrosine (nTyr)-protein adducts is a reliable biomarker of ONOO− generation. Therefore, the present study was aimed at investigating the role of endogenous nitric oxide in regulating Na+,K+-ATPase activity in the kidney, and at investigating the possible contribution of reactive nitrogen species (RNS) by measuring of iNOS activity. In addition, the present study was aimed at investigating the relationship between nTyr formation with iNOS and Na+,K+-ATPase activities. Previously in our study, nTyr was not detectable in kidney of normal control animals but was detected markedly in LPS exposed animals. In this study, kidney Na+,K+-ATPase activity were maximally inhibited 6 h after LPS injection (P:0.000) and LPS treatment significantly increased iNOS activity of kidney (P:0.000). The regression analysis revealed a very close correlation between Na+,K+-ATPase activity and nTyr levels of LPS treated animals (r = −0.868, P = 0.001). Na+,K+-ATPase activity were also negatively correlated with iNOS activity (r = −0.877, P = 0.001) in inflamed kidney. These data suggest that NO• and ONOO− contribute to the development of oxidant injury. Furthermore, the source of NO• may be iNOS. iNOS are expressed by the kidney, and their activity may increase following LPS administration. In addition, NO• and ONOO− formation inhibited Na+,K+-ATPase activity. This results also have strongly suggested that bacterial LPS disturbs activity of membrane Na+,K+-ATPase that may be an important component leading to the pathological consequences such as renal dysfunction in which the production of RNS are increased as in the case of LPS challenge. (Mol Cell Biochem 271: 107–112, 2005)
Similar content being viewed by others
References
Olsson LE, Wheeler MA, Sessa WC, Weiss RM: Bladder instillation and intraperitoneal injection of Escherichia coli lipopolysaccharide up-regulate cytokines and iNOS in rat ürinary bladder. J Pharm Exp Ther 284: 1203–1208, 1998
Essani NA, McGruice GM, Manning AM, Jaeschke H: Differential induction of mRNA for ICAM-1 and selectins in hepatocytes, kuppfer cells and endothelial cells during endotoxemia. Biochem Biophys Res Commun 221: 74–82, 1995
Takahashi M, Ogasawara K, Takeda K, Hashimoto W, Sakihara H, Kumagai K, Anzai R, Satoh M, Seki S: LPS induces NK1.1+alpha beta T cells with potent cytotoxicity in the liver of mice via production of IL-12 from Kuppfer cells. J Immunol 156: 2436–2442, 1996
Schwartz D, Mendonca M, Schwartz I, Xia Y, Satriano J, Wilson CB, Blantz RC: Inhibition of constitutive nitric oxide synthase (NOS) by nitric oxide generated by inducible NOS after lipopolysaccharide administration provokes renal dysfunction in rats. J Clin Invest 100: 439–448, 1997
Markewitz BA, Michael JR, Kohan DE: Cytokine-induced expression of a nitric oxide synthase in rat renal tubule cells. J Clin Invest 91: 2138–2143, 1993
Zurovsky Y, Gispaan I: Antioxidants attenuate endotoxin-induced acute renal failure in rats. Am J Kidney Dis 25: 51–57, 1995
Shultz PJ, Raij L: Endogenously synthesized nitric oxide prevents endotoxin-induced glomerular thrombosis. J Clin Invest 90: 1718–1725, 1992
Guzman NJ, Fang MZ, Tang SS, Ingelfinger JR, Garg LC: Autocrine inhibition of Na+K+ATPase by nitric oxide in mouse proximal tubule epithelial cells. J Clin Invest 95: 2083–2088, 1995
Kang YH, Falk MC, Bentley TB, Lee CH: Distriubution and role of lipopolysaccharide in the pathogenesis of acute renal proximal tubule injury. Shock 4: 441–449, 1995
Zhang C, Walker LM, Mayeux PR: Role of nitric oxide in lipopolysaccharide-induced oxidant stres in the rat kidney. Biochem Pharmacol 59: 203–209, 2000
Fukuyama N, Takebayashi Y, Hida M, Ishida H, Ichimori K, Nakazawa H: Clinical evidence of peroxynitrite formation in chronic renal failure patients with septic shock. Free Radic Biol Med 22: 771–774, 1997
Bian K, Davis K, Kuret J, Binder L, Murad F: Nitrotyrosine formation with endotoxin-induced kidney injury detected by immunohistochemistry. Am J Physiol 277: F33–F40, 1999
Mishra OP, Delivoria-Papadopoulos M, Cahilane G, Wagerle LC: Lipid peroxidation as the mechanism of modification of the affinity of the Na+,K+-ATPase active sites for ATP, K+,Na+, and strophanthidin in vitro. Neurochem Res 14: 845–851, 1989
Sato T, Kamata Y, Irifune M, Nishikawa T: Inhibitory effect of several nitric oxide-generating compounds on purified Na+,K+-ATPase activity from porcine cerebral cortex. J Neurochem 68: 1312–1318, 1997
Qayyum I, Zubrow AB, Ashraf QM, Kubin J, Delivoria-Papadopoulos M, Mishra OP: Nitration as a mechanism of Na+,K+-ATPase modification during hypoxia in the cerebral cortex of the guinea pig fetus. Neurochem Res 26: 1163–1169, 2001
Türközkan N, Ünlü A, Ertabak A, Cimen B, Karabicak U: The effects of peroxynitrite on erythrocytes. Clin Chem Lab Med 39: 1263–1266, 2001
Zhang C, Imam SZ, Ali SF, Mayeux PR: Peroxynitrite and the regulation of Na+,K+-ATPase activity by angiotensin II in the rat proximal tubule. Nitric Oxide 7: 30–35, 2002
Muriel P, Sandoval G: Nitric oxide and peroxynitrite anion modulate liver plasma membrane fluidity and Na+,K+-ATPase activity. Nitric Oxide 4: 333–342, 2000
Kang DG, Kim JW, Lee J: Effects of nitric oxide synthesis inhibition on the Na,K-ATPase activity in the kidney. Pharm Res 41: 121–125, 2000
Unlu A, Türközkan N, Cimen B, Karabiçak U, Yaman H: The effect of E. coli-derived lipopolysaccharides on plasma levels of malondialdehyde and 3-nitrotyrosine. Clin Chem Lab Med 39: 491–493, 2001
Cimen B, Türközkan N, Seven I, Unlü A, Karasu C: Impaired Na+K+ATPase activity as a mechanism of reactive nitrogen species-induced cytotoxicity in guinea pig liver exposed to lipopolysaccharides. Mol Cell Biochem 2004
Ertabak A, Kutluay T, Unlu A, Türközkan N, Cimen B, Yaman H: The effects of desferrioxamine on peroxynitrite-induced oxidative damage in erythrocytes. Cell Biochem Funct 2004
Serpersu E, Ciliv G: Some properties of (Na+K+)-dependent adenosinetriphosphatase from human erythrocytes. Biochem Med 20: 31–39, 1978
Feelisch M. Noack EA: Correlation between nitric oxide formation during degradation of organic nitrates and activation of guanylate cyclase. Eur J Pharmacol 139: 19–30, 1987
Knowles RG, Marrett M, Salter M, Moncada S: Differential induction of brain, lung and liver nitric oxide synthase by endotoxin in the rat. Biochem J 270: 833–836, 1990
Morikawa A, Kato Y, Sugiyama T, Koide N, Chakravortty D, Yoshida T, Yokochi T: Role of nitric oxide in lipopolysaccharide-induced hepatic injury in D-galactosamine-sensitized mice as an experimental endotoxic shock model. Infect Immun 67: 1018–1024, 1999
Knotek M, Rogachev B, Wang W, Ecder T, Melnikov V, Gengaro PE, Esson M, Edelstein CL, Dinarello CA, Schrier RW: Endotoxemic renal failure in mice: Role of tumor necrosis factor independent of inducible nitric oxide synthase. Kidney Int 59: 2243–2249, 2001
Liu S, Adcock IM, Old RW, Barnes PJ, Evans TW: Lipopolysaccharide treatment in vivo induces widespread tissue expression of inducible nitric oxide synthase mRNA. Biochem Biophys Res Commun 196: 1208–1213, 1993
Yang F, Comtois AS, Fang L, Hartman NG, Blaise G: Nitric oxide derived-nitrate anion contributes to endotoxic shock and multiple organ injury/dysfunction. Crit Care Med 30: 650–657, 2002
Paya D, Stoclet JC: Involvement of bradykinin and nitric oxide in the early hemodynamic effects of lipopolysaccharide in rats. Shock 3: 376–379, 1995
Morrissey JJ, McCracken R, Kaneto H, Vehaskari M, Montani D, Klahr S: Location of an inducible nitric oxide synthase mRNA in the normal kidney. Kidney Int 45: 998–1005, 1994
Mayeux PR, Garner HR, Gibson JD, Beanum VC: Effect of lipopolysaccharide on nitric oxide synthase activity in rat proximal tubules. Biochem Pharm 49: 115–118, 1995
Watanabe N, Miura S, Zeki S, Ishii H: Hepatocellular oxidative DNA injury induced by macrophage-derived nitric oxide. Free Radic Biol Med 30: 1019–1028, 2000
D’Ambrossio SM, Gibson-D’Ambrossio RE, Brady T, Oberyszyn AS., Robertson FM: Mechanisms of nitric oxide-induced cytotoxicity in normal human hepatocytes. Environ Mol Mutagen 37: 46–54, 2001
Nussler AK, Billiar TR: Inflammation, immunoregulation and inducible nitric oxide synthase. J Leuk Biol 54: 171–178, 1993
Sass G, Koerber K, Bang R, Guehring H, Tiegs G: Inducible nitric oxide synthase is critical for immune-mediated liver injury in mice. J Clin Invest 107: 439–447, 2001
Kurose I, Miura S, Higuchi H, Watanabe N, Kamegaya Y, Takaishi M, Tomita K, Fukumura D, Kato S, Ishii H: Increased nitric oxide synthase activity as a cause of mitochondrial dysfunction in rat hepatocytes: Roles for tumor necrosis factor-alpha. Hepatology 24: 1185–1192, 1996
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seven, I., Türközkan, N. & Çimen, B. The effects of nitric oxide synthesis on the Na+,K+-ATPase activity in guinea pig kidney exposed to lipopolysaccharides. Mol Cell Biochem 271, 107–112 (2005). https://doi.org/10.1007/s11010-005-5616-1
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11010-005-5616-1