Abstract
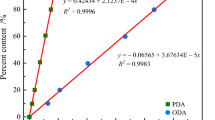
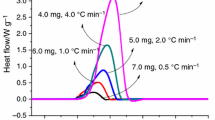
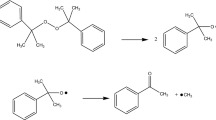
Diallyl phthalate (DAP), a crucial raw material in producing resin materials, has been studied widely in terms of its polymerization process and application in material modification. However, less attention has been paid to its safety. In this study, the influence of initiators on the thermal hazard of DAP was examined from various viewpoints by conducting thermogravimetric and differential scanning calorimetry experiments and by performing thermokinetic analysis with multiple models. Moreover, the reaction mechanism of DAP was studied. The three-step autocatalysis reaction simulations agreed well with the experimental findings obtained for DAP. The simulated conversion limit time and time to the maximum rate under adiabatic conditions indicate that the thermal hazard of DAP can serve as a reference for controlling its production and storage temperatures.












Similar content being viewed by others
Abbreviations
- β :
-
Heating rate/°C min−1
- T o :
-
Apparent onset temperature /°C
- T p :
-
Peak temperature/°C
- T e :
-
End set temperature/°C
- T eo :
-
Temperature of the extrapolated onset/°C
- ΔH :
-
Heat of reaction of DSC/J g−1
- α :
-
Extent of conversion
- A :
-
Frequency factor
- E a :
-
Apparent activation energy/kJ mol−1
- R :
-
Universal gas constant/8.314 J mol−1K−1
- C s :
-
Constant
- i :
-
Approximate values obtained from integral temperature
- B :
-
Approximate values obtained from integral temperature
- G(α):
-
Integral form of the mechanism function
- k 0 :
-
Pre-exponential factor
- n :
-
Reaction orders
- z :
-
Represents the autocatalytic constant
- γ :
-
Degree conversion of ratio
- TCL :
-
Conversion limit time/day
- TMR ad :
-
Time to the maximum rate at adiabatic conditions/day
- TRI :
-
Thermal risk index
- ρ :
-
Measurement of the severity of the reaction
- ε :
-
Measurement of the probability of reaction occurrence
References
Liang YY, Zhou XP, Liao YG, Wu J, Xie XL, Zhou HM. Reactive polycarbonate/diallyl phthalate blends with high optical transparency, good flowability and high mechanical properties. Polym. 2016;91:89–97.
Jo Y, Choe Y. Mechanical properties of core-shell rubber (CSR)/diallyl phthalate (DAP)/epoxy systems for electronic packaging materials. Mol Cryst Liq Cryst. 2011;539(1):190–5.
Sato T, Nomura K, Hirano T, Seno M. Initiator-fragment incorporation radical polymerization of diallyl phthalate: kinetics, formation of hyperbranched polymer, and iridescent porous film thereof. J Appl Polym Sci. 2006;102(1):408–15.
Gustin JL. Understanding vinyl acetate polymerization accidents. Org Process Res Dev. 2005;12(6):36–46.
Casson V, Snee T, Maschio G. Investigation of an accident in a resins manufacturing site: the role of accelerator on polymerisation of methyl methacrylate. J Hazard Mater. 2014;270:45–52.
Xu SA, He M, Shi QF, Jin GC, Yao JQ, Yu RB, et al. Effect of ultrasonic separation on the structure and properties of diallyl phthalate prepolymer. Ultrason Sonochem. 2008;15(4):364–9.
Chou HC, Wu SH, Chiang CC, Horng JJ, Chi JH, Shu CM. Effect of stiring rate for thermal runaway reaction in cumene hydroperoxide manufacturing process using caloeimetric techniques. J Therm Anal Calorim. 2011;106:243–8.
Zhu Y, Liang C, Bo Y, Xu S. Compatibilization of polypropylene/recycled polyethylene terephthalate blends with maleic anhydride grafted polypropylene in the presence of diallyl phthalate. J Polym Res. 2015;22(3):1–12.
He SB, Liang GZ, Wang JH, Yan HX. Mechanical and thermal properties of bisphenol A-based cyanate ester and diallyl phthalate blends. Polym Bull. 2008;62(2):237–46.
Xie LJ, Jiang JC, Huang AC, Tang Y, Liu YC, Zhou HL, et al. Calorimetric evaluation of thermal stability of organic liquid hydrogen storage materials and metal oxide additives. Energies. 2022;15(6):2236.
Kuang YC, He BS, Wang CJ, Tong WX, He D. Thermogravimetric analysis of pulverized coal in low oxygen atmosphere. Thermochim Acta. 2021;703:178992.
Vyazovkin S. Kissinger method in kinetics of materials: things to beware and be aware of. Mol. 2020;25(12):2813.
Sinditskii VP, Smirnova AD, Serushkin VV, Yudin NV, Vatsadze IA, Dalinger IL, et al. Nitroderivatives of N-pyrazolyltetrazoles: thermal decomposition and combustion. Thermochim Acta. 2021;698:178876.
Wu ZH, Huang AC, Tang Y, Yang YP, Liu YC, Li ZP, et al. Thermal effect and mechanism analysis of flame-retardant modified polymer electrolyte for lithium-ion battery. Polym (Basel). 2021;13(11):1675.
Yang YP, Jiang JC, Huang AC, Tang Y, Liu YC, Xie LJ, et al. 3-(Trifluoromethyl) benzoylacetonitrile: a multi-functional safe electrolyte additive for LiNi0.8Co0.1Mn0.1O2 cathode of high voltage lithium-ion battery. Process Saf Environ Prot. 2022;160:80–90.
Sun Y, Papadaki M, Jiao Z, Zhu W, Jiang JC, Mashuga C, et al. Reaction hazard and mechanism study of H2O2 oxidation of 2-butanol tomethyl ethyl ketone using DSC, Phi-TEC II and GC-MS. J Loss Prev Process Ind. 2020;66:104177.
Fang SW, Lin Y, Huang Z, Huang HY, Chen S, Ding LX. Investigation of co-pyrolysis characteristics and kinetics of municipal solid waste and paper sludge through TG-FTIR and DAEM. Thermochim Acta. 2021;700:178889.
Li ZP, Jiang JC, Huang AC, Tang Y, Miao CF, Zhai J, et al. Thermal hazard evaluation on spontaneous combustion characteristics of nitrocellulose solution under different atmospheric conditions. Sci Rep. 2021;11(1):24053.
Gözke G, Açıkalın K. Pyrolysis characteristics and kinetics of sour cherry stalk and flesh via thermogravimetric analysis using isoconversional methods. J Therm Anal Calorim. 2020;146(2):893–910.
Li P, Yang YL, Li JH, Miao GD, Zheng KY, Wang YH. Study on the oxidation thermal kinetics of the spontaneous combustion characteristics of water-immersed coal. Thermochim Acta. 2021;699:178914.
Xiao Y, Zhang H, Liu KH, Shu CM. Macrocharacteristics and the inhibiting effect of coal spontaneous combustion with various treatment durations of ionic liquids. Thermochim Acta. 2021;703:179012.
Huang AC, Huang CF, Xing ZX, Jiang JC, Shu CM. Thermal hazard assessment of the thermal stability of acne cosmeceutical therapy using advanced calorimetry technology. Process Saf Environ Prot. 2019;131:197–204.
Xia Y, Huang Y, Li Y, Liao S, Long Q, Liang J. LaPO4: Ce, Tb, Yb phosphor—synthesis and kinetics study for thermal process of precursor by Vyazovkin, OFW, KAS, Starink, and Mastplosts methods. J Therm Anal Calorim. 2015;120(3):1635–43.
Alves JLF, da Silva JCG, Mumbach GD, Domenico MD, Bolzan A, Machado RAF, et al. Evaluating the bioenergy potential of cupuassu shell through pyrolysis kinetics, thermodynamic parameters of activation, and evolved gas analysis with TG/FTIR technique. Thermochim Acta. 2022;711:179187.
Alavi SE, Abdoli MA, Khorasheh F, Bayandori MA. Non-isothermal pyrolysis of used lubricating oil and the catalytic effect of carbon-based nanomaterials on the process performance. J Therm Anal Calorim. 2019;139(2):1025–36.
Xia ZY, Wu WQ, Chen WH, Chen LP, Guo ZC. Thermal decomposition kinetics of three anthraquinone hazardous waste. Thermochim Acta. 2021;697:178852.
Wu H, Yang N, Tang Y, Jiang JC, Huang AC. Thermal stability evaluation of T152 emulsifier on the modification influence of fireworks propellant. Processes. 2022;10(8):1606.
Berčič G, Djinović P, Pintar A. Simplified approach to modelling the catalytic degradation of low-density polyethylene (LDPE) by applying catalyst-free LDPE-TG profiles and the Friedman method. J Therm Anal Calorim. 2018;136(3):1011–20.
Zhang Z, Chen J, Liu H, Xiao C. Applicability of Kissinger model in nonisothermal crystallization assessed using a computer simulation method. J Therm Anal Calorim. 2014;117(2):783–7.
Zhou HL, Jiang JC, Huang AC, Tang Y, Zhang Y, Huang CF, et al. Calorimetric evaluation of thermal stability and runaway hazard based on thermokinetic parameters of O, O–dimethyl phosphoramidothioate. J Loss Prev Process Ind. 2022;75:104697.
Liu YC, Jiang JC, Huang AC, Tang Y, Yang YP, Zhou HL, et al. Hazard assessment of the thermal stability of nitrification by-products by using an advanced kinetic model. Process Saf Environ Prot. 2022;160:91–101.
Liu YC, Huang AC, Tang Y, Huang CF, Shen Q, Shu CM, et al. Thermokinetic analysis of the stability of acetic anhydride hydrolysis in isothermal calorimetry techniques. J Therm Anal Calorim. 2021;147(14):7865–73.
Liu SH, Cao CR, Lin WC, Shu CM. Experimental and numerical simulation study of the thermal hazards of four azo compounds. J Hazard Mater. 2019;365:164–77.
Liu SH, Cao CR, Shu CM. Using thermal analysis with kinetic calculation method to assess the thermal stability of 2-cyanopropan-2-yliminourea. J Loss Prev Process Ind. 2020;64:104084.
Stoessel F. Thermal safety of chemical processes: risk assessment and process design. Org Process Res Dev. 2008;7(3):351–3.
Wang Q, Rogers WJ, Mannan MS. Thermal risk assessment and rankings for reaction hazards in process safety. J Therm Anal Calorim. 2009;98(1):225–33.
Acknowledgements
This study was supported by the National Nature Science Foundation of China (No. 21927815), the National Key Research Development Program of China (No. 2021YFC3001203).
Author information
Authors and Affiliations
Contributions
H-L Z and A-C H performed the analysis and wrote the paper; Y T contributed the literature research; J Z and Y-C L ofered the methodology; Y-C C and C-M S conceived the research theme; Z-X X and J-C J carry out writing-review and supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, HL., Liu, YC., Tang, Y. et al. Utilizing thermokinetic and calorimetric methods to assess the impact of an initiator on the thermal hazard of diallyl phthalate. J Therm Anal Calorim 148, 5017–5027 (2023). https://doi.org/10.1007/s10973-022-11819-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11819-1