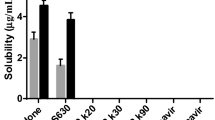
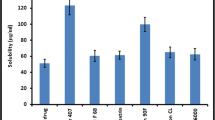
Abstract
The acquired immunodeficiency syndrome remains a major problem in worldwide public health and its antiretroviral treatment therapy combines at least three high activity classes of drugs. Access to antiretroviral treatment for HIV-infected patients is a global public health priority and efavirenz (EFZ) is one of the first drug choices. However, EFZ is classified as a class II drug, according to the biopharmaceutics classification system due to low solubility and high permeability, which leads it to fail in absorption and hence bioavailability. One of the approaches used to overcome these issues is the preparation of solid dispersions. In this study, thermal analysis and pyrolysis coupled with gas chromatography-mass spectrometry (GC/MS) were employed to determine efavirenz thermal stability and the solid dispersion with poly (vinylpyrrolidone-co-vinylacetate) (PVPVA 64). Thus, it suggests that EFZ was converted to its amorphous state, and there was an increase in EFZ thermal stability when it is dispersed in a polymer matrix compared to the drug alone, since there was an increase in the activation Energy (Ea) and a decrease in frequency factor (A). Furthermore, there was a change in the zero reaction order to one. The thermal stability time of the formulation was estimated at 7 months. In addition, it was possible to observe a good correlation between loss of mass and the technique of Pyrolysis-GC–MS and to posit the formation of new fragments, which clarifies the technique with regard to the identification of thermal decomposition fragments.
















Similar content being viewed by others
References
Madhavi BB, Kusum B, Chatanya CHK, Madhu MN, Harsha VS, Banji D. Dissolution enhancement of efavirenz by solid dispersion and PEGylation techniques. Int J Pharm Investig. 2011. https://doi.org/10.4103/2230-973X.76726.
Sathigari S, Chada G, Lee YHP, Wright N, Parsons DL, Rangari VK, Fasina O, Babu RJ. Physicochemical characterization of efavirenz-cyclodextrin inclusion complexes. AAPS PharmSciTech. 2009. https://doi.org/10.1208/s12249-008-9180-3.
Sharma A, Jain CP. Solid dispersion: a promising technique to enhance solubility of poorly water solubledrug. Int J Drug Deliv. 2011;3:149–70.
Dinunzio JC, Brough C, Miller DA, Williams RO III, McGinity JW. Applications of KinetiSol® dispersing for the production of plasticizer free amorphous solid dispersions. Eur J Pharm Sci. 2010. https://doi.org/10.1016/j.ejps.2010.03.002.
Tiwari R, Tiwari G, Srivastava B, Rai AK. Solid dispersions: an overview to modify bioavailability of poorly water soluble drugs. Int J PharmTech Res. 2009;1:1338–49.
Oliveira MA, Yoshida MI, Gomes ECL. Análise térmica aplicada a fármacos e formulações farmacêuticas na indústria farmacêutica. Quim Nova. 2011;34:1224–30.
Baird JA, Taylor LS. Evaluation of amorphous solid dispersion properties using thermal analysis techniques. Adv Drug Deliv Rev. 2012. https://doi.org/10.1016/j.addr.2011.07.009.
Sovizi MR, Hosseini SG. Studies on the thermal behavior and decomposition kinetic of drugs cetirizine and simvastatin. J Therm Anal Calorim. 2013. https://doi.org/10.1007/s10973-012-2651-5.
Costa SPM, Silva KER, Medeiros GCR, Rolim LA, Oliveira JF, Lima MCA, Galdino SL, Pitta IR, Rolim-Neto PJ. Thermal behavior and compatibility analysis of the new chemical entity LPSF/FZ4. Thermochim Acta. 2013. https://doi.org/10.1016/j.tca.2013.03.003.
Fandaruff C, Araya-Sibaja AM, Pereira RN, Hoffmeister CRD, Rocha HVA, Silva MAS. Thermal behavior and decomposition kinetics of efavirenz under isothermal and non-isothermal conditions. J Therm Anal Calorim. 2014. https://doi.org/10.1007/s10973-013-3306-x.
Araújo AAS, Mercuri LP, Seixas SRS, Storpitis S, Matos JR. Determinação dos teores de umidade e cinzas de amostras comerciais de guaraná utilizando métodos convencionais e análise térmica. Ver. Bras. Cienc. Farm. 2006. https://doi.org/10.1590/S1516-93322006000200013.
Storpirtis S, Gonçalves JE, Chiann C, Gai MN. Ciências Farmacêuticas: Biofarmacotécnica. Rio de Janeiro: Guanabara Koogan; 2009.
Klancnik G, Medved J, Mrvar P. Differential thermal analysis (DTA) and differential scanning calorimetry (DSC) as a method of material investigation, RMZ. Mater Geoenviron. 2010;57:127–42.
Macêdo RO, Nascimento TG. Thermal characterization of lapachol by means of TG and DSC coupled to a photovisual system. J Therm Anal Calorim. 2001. https://doi.org/10.1023/A:1011552613564.
Silva RMF, Medeiros FPM, Nascimento TG, Macêdo RO, Rolim-Neto PJ. Thermal characterization of Indinavir sulfate using TG, DSC and DSC-photovisual. J Therm Anal Calorim. 2009. https://doi.org/10.1007/s10973-008-8912-7.
Viana OS, Araujo AAS, Simões RA, Soares JL, Matos CRS, Grangeiro SC Jr, Lima M, Rolim-Neto PJ. Kinetic analysis of the thermal e composition of efavirenz and compatibility studies with selected excipients. Lat Am J Pharm. 2008;27:211–6.
Brown ME. Introduction to the thermal analysis. Techniques and applications. 2nd ed. Dordrecht: Kluwer Academic Publishers; 2001.
Jankovic B, Mentus S, Jankovic M. A kinetic study of the termal decomposition process of potassium metabisulfite: estimation of distributed reactivity model. J Phys Chem Solids. 2008;69:1923–33.
Moura EA, Correia LP, Pinto MF, Procópio JVV, Souza FS, Macêdo RO. Thermal characterization of the solid state and raw material fluconazole by thermal analysis and pyrolysis coupled to GC/MS. J Therm Anal Calorim. 2010. https://doi.org/10.1007/s10973-009-0473-x.
Oliveira AH, Moura EA, Pinto MF, Procópio JVV, Souza VG, Souza FS, Macêdo RO. Thermal characterization of raw material pentoxifylline using thermoanalytical techniques and Pyr-GC/MS. J Therm Anal Calorim. 2011. https://doi.org/10.1007/s10973-011-1839-4.
Boer TM, Procópio JVV, Nascimento TG, Macêdo RO. Correlation of thermal analysis and pyrolysis coupled to GC–MS in the characterization of tacrolimus. J Pharm Biomed Anal. 2013. https://doi.org/10.1016/j.jpba.2012.01.040.
Kolter K, Karl M, Gryczke A. Hot-melt extrusion with BASF pharma polymers: extrusion compendium 2nd revised and enlarged edition. BASF SE Pharma Ingredients & Services. 2012. http://www.pharmaingredients.basf.com/Documents/ENP/Brochure/EN/03_120803%20Hot%20Melt%20Extrusion%20with%20BASF%20Pharma%20Polymers.pdf. Accessed 25 Feb 2015.
Maurin MB, Roew SM, Blom K, Pierce ME. Kinetics and mechanism of hydrolysis of efavirenz. Pharm Res. 2002. https://doi.org/10.1023/A:1015160132290.
Beyler CL, Hirschler MM. Thermal decomposition of polymers. In: DiNenno PJ, editor. SFPE handbook of fire protection engineering. Bethesda, MA: Springer; 2002. p. 110–31.
Macêdo RO, Nascimento TG. Quality control of thiabendazole pre-formulation and tablets by TG and DSC coupled to the photovisual system. Thermochim Acta. 2002. https://doi.org/10.1016/S0040-6031(02)00088-6.
Teja SB, Patil SP, Shete G, Patel S, Bansal AK. Drug-excipient behavior in polymeric amorphous solid dispersions. J Excip Food Chem. 2013;4:70–94.
Tita B, Jurca T, Tita D. Thermal stability of pentoxifylline: active substance and tablets. Part 1. Kinetic study of the active substance under non-isothermal conditions. J Therm Anal Calorim. 2013. https://doi.org/10.1007/s10973-013-3118-z.
Mohamed MA, Attia AK. Thermal behavior and decomposition kinetics of cinnarizine under isothermal and non-isothermal conditions. J Therm Anal Calorim. 2016. https://doi.org/10.1007/s10973-016-5551-2.
Bianchi O. Avaliação da Degradação Não-Isotérmica de Madeira Através de Termogravimetria-TGA. Polímeros. 2010.
Acknowledgements
The authors would like to thank the Improving Coordination for Senior Staff (CAPES) and National Council of Scientific and Technological Development (CNPq) for financial support, Pharmaceutical Laboratory in the State of Pernambuco (LAFEPE) and the University of Toronto for the donation of raw materials and the Department of Pharmaceutical Sciences of the Federal University of Paraíba for performing the Pyrolysis-GC/MS analyses and DSC photo-visuals.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Costa, S.P.M., de Andrade Gomes, T., da Silva, K.E.R. et al. Characterization and thermal stability study of efavirenz and solid dispersion with PVPVA 64 by means of thermal analysis and pyrolysis coupled with GC/MS. J Therm Anal Calorim 146, 2169–2182 (2021). https://doi.org/10.1007/s10973-020-10350-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-10350-5