Abstract
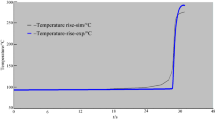
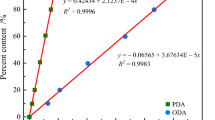
As a special engineering plastics with excellent comprehensive properties, polyarylether (PAE) has been widely used in automobile manufacturing, aerospace, electronic communications, mechanical manufacturing and other fields. In industry, PAE is obtained by polymerization reaction. However, a number of accidents were caused by polymerization reactions. Therefore, a series of experiments were carried out to investigate the thermal hazard of PAE polymerization process to prevent the polymerization accidents. First, the reaction calorimeter (RC1e) was used to measure the heat released in the reaction process to obtain the thermodynamic parameters. The RC1e results showed that the adiabatic temperature rise (ΔTad) of polymerization was 97.99 °C, the maximum temperature attained by synthesis reaction was 127.99 °C and the maximum temperature technical for reasons was 110.60 °C. Then, the pyrolysis characteristics of the PAE in air and nitrogen atmosphere at different heating rates were scanned via thermogravimetry, and the apparent activation energy was calculated by Starink method. The PAE showed two significant mass loss peaks in the air atmosphere, but only one in the nitrogen atmosphere. The pyrolysis of PAE in the air atmosphere was more thoroughly compared to that in the nitrogen atmosphere. Finally, the risk class of thermal runaway of polymerization was evaluated according to the relevant criteria, the severity was “class 2,” and the risk class was “class 3.” These results were the foundation for preventive measures to reduce the potential risk during polymerization of PAE.










Similar content being viewed by others
Abbreviations
- β :
-
Heating rate (K min−1)
- C :
-
Constant
- C p :
-
Specific heat capacity (J K−1 mol −1)
- E a :
-
The apparent activation energy (kJ·mol−1)
- ΔH :
-
Reaction enthalpy (J mol−1)
- MTT :
-
Maximum temperature for technical reasons (°C)
- MTSR :
-
Maximum temperature attained by synthesis reaction (°C)
- T :
-
Temperature (°C)
- ΔT ad :
-
Adiabatic temperature rise (°C)
- T cf :
-
Cooling failure temperature (°C)
- TMRad :
-
Time to maximum rate under adiabatic decomposition conditions (°C)
- T D24 :
-
Temperature at which time to maximum rate is 24 h (°C)
- T r :
-
Reaction temperature (°C)
- T p :
-
Process temperature (°C)
- R :
-
Ideal gas constant (8.314 J mol−1 K−1)
- X :
-
Heat accumulation degree (%)
References
Mihailidou EK, Antoniadis KD, Assael MJ. The 319 major industrial accidents since 1917. Int Rev Chem Eng. 2012;4:529–40.
André R, Giordano M, Mathona C, Naumann R. A new reaction calorimeter and calorimetric tools for safety testing at laboratory scale. Thermochim Acta. 2003;405:43–50.
Sun Q, Jiang L, Li M, Sun J. Assessment on thermal hazards of reactive chemicals in industry: state of the art and perspectives. Prog Energy Combust Sci. 2020;78:100832.
Lin Z, Wen Z, Maria IP, Mannan MS, Mustafa A. Probing into styrene polymerization runaway hazards: effects of the monomer mass fraction. ACS Omega. 2019;4:8136–45.
Saada R, Patel D, Saha B. Causes and consequences of thermal runaway incidents-will they ever be avoided? Process Saf Environ Prot. 2015;97:109–15.
Hugo P, Steinbach J. A comparison of the limits of safe operation of a SBR and a CSTR. Chem Eng Sci. 1986;41:1081–7.
Maestri F, Rota R. Temperature diagrams for preventing decomposition or side reactions in liquid–liquid semibatch reactors. Chem Eng Sci. 2006;61:3068–78.
Steensma M, Westerter R. Thermally safe operation of a cooled semi-batch reactor. Slow liquid–liquid reactions. Chem Eng Sci. 1988;43:2125–32.
Gygax R. Chemical reaction engineering for safety. Chem Eng Sci. 1988;8:1759–71.
Stoessel F. Thermal safety of chemical processes: risk assessment and process design. 1st ed. Weinheim: Wiley; 2008.
Am Ende DJ, Clifford PJ, Northrup DL. The role of reaction calorimetry in the development and scale-up of aromatic nitrations. Thermochim Acta. 1996;289(2):143–54.
Clark JD, Shah AS, Peterson JC, Grogan FM, Camden SK. Application of reaction calorimetry toward understanding the large scale chemistry of ethyl diazoacetate. Thermochim Acta. 2001;367:75–84.
Lerena P, Wehner W, Weber H, Stoessel F. Assessment of hazards linked to accumulation in semi-batch reactors. Thermochim Acta. 1996;289:127–42.
Nogent H, Le Tacon X. The differential reaction calorimeter: examples of use. J Loss Prev Process Ind. 2003;16(2):133–9.
Garron A, Swierczynski D, Bennici S, Auroux A. New insights into the mechanism of H2 generation through NaBH4 hydrolysis on Co-based nanocatalysts studied by differential reaction calorimetry. Int J Hydrog Energy. 2009;34:1185–99.
Das PK. Theoretical estimation of adiabatic temperature rise from the heat flow data obtained from a reaction calorimeter. Thermochim Acta. 2012;530:17–24.
Zhang L, Yu WD, Pan XH, Fang JJ, Hua M, Chen FM, Jiang JC. Thermal hazard assessment for synthesis of 3-methylpyridine-N-oxide. J Loss Prev Process Ind. 2015;35:316–20.
Ben TI, Balland L, Bensahla N, Nordine M. Thermokinetic parameter determination of methacrylates radical polymerization by using real-time reaction calorimetry. J Therm Anal Calorim. 2017;130:2341–9.
Hua M, Liang X, Wei CY, Zhang L, Pan X, Jiang J, Ni L, Jiang J. Inherent safer design for chemical process of 1,4-dioldiacetate-2-butene oxidized by ozone. Chem Eng Commun. 2019. https://doi.org/10.1080/00986445.2019.1657420.
Fila K, Gargol M, Goliszek M, Podkościelna B. Synthesis of epoxy resins derivatives of naphthalene-2,7-diol and their cross-linked products. J Therm Anal Calorim. 2019;138:4349–58.
Alonso A, Lázaro M, Lázaro P, Lázaro D, Alvear D. LLDPE kinetic properties estimation combining thermogravimetry and differential scanning calorimetry as optimization targets. J Therm Anal Calorim. 2019;138:2703–13.
Tseng JM, Wu TC, Hsieh TF, Huang PJ, Lin CP. The thermal hazard evaluation of 1,1-di(tert-butylperoxy) cyclohexane by DSC using non-isothermal and isothermal-kinetic simulations. J Loss Prev Process Ind. 2012;25:703–8.
Yu AD, Cao CR, Pan XH, Shu CM, Wang WJ. Solid thermal explosion of autocatalytic material based on nonisothermal experiments: multistage evaluations for 2,2′-azobis(2-methylpropionitrile) and 1,1′-azobis (cyclohexanecarbonitrile). Process Saf Prog. 2019;38:10–9.
Wei CY, Lin WC, Pan XH, Shu CM, Hua M, Jiang HC, Jiang JC. Thermal risk assessment of tert-butylperoxy-2-ethylhexyl carbonate for storage and transport. J Therm Anal Calorim. 2019;138:2891–900.
Wang WJ, Fang JJ, Pan XH, Hua M, Jiang JJ, Ni L, Jiang JC. Thermal research on the uncontrolled behavior of styrene bulk polymerization. J Loss Prev Process Ind. 2019;57:239–44.
Liu SH, Zhan XB, Lu YM, Xu ZL, Wu T. Thermal hazard evaluation of AIBME by micro-calorimetric technique coupled with kinetic investigation. J Therm Anal Calorim. 2020;141:1443–52.
Zhang T, Wang J, Derradji M, Ramdani N, Wang H, Lin ZW, Liu W. Synthesis, curing kinetics and thermal properties of a novel self-promoted fluorene-based bisphthalonitrile monomer. Thermochim Acta. 2015;602:22–9.
Yu X, Shang Z, Zhang K. Thermally stable polybenzoxazines via tetrahydrophthalimide-functional monobenzoxazines: synthesis, characterization and thermally activated polymerization kinetics. Thermochim Acta. 2019;675:29–37.
Liang XM, Cheng YC, Lin WC, Tung PH, Huang HQ, Pan X, Shu CM, Jiang J. Analysis and characterisation of 1-butyl-3-methylimidazolium hexafluorophosphate as a humectant of nitrocellulose. J Mol Liq. 2020;303:112617.
Jiang HC, Lin WC, Hua M, Pan XH, Shu CM, Jiang JC. Analysis of thermal stability and pyrolysis kinetic of dibutyl phosphate-based ionic liquid through thermogravimetry, gas chromatography/mass spectrometry, and Fourier transform infrared spectrometry. J Therm Anal Calorim. 2019;138:489–99.
https://www.mem.gov.cn/gk/gwgg/agwzlfl/yj_01/201701/t20170112_242237.shtml.
Jiang JC, Jiang W, Ni L, Zhang WX, Zou MY, Shen SL, Pan Y. The modified Stoessel criticality diagram for process safety assessment. Process Saf Environ Prot. 2019;129:112–8.
Nanchen A, Steinkrauss M, Stoessel F. Utilisation of the criticality classes within TRAS410. Forsch Ingenieurwes. 2009;73(1):3–10.
Acknowledgements
The authors are grateful for the financial support of the National Key Research and Development Program (2016YFC0801500), the Major Program of the National Natural Science Foundation of China (21436006, 51874181, 51834007, 51804167), the Major Projects of the Natural Science Research for Colleges in Jiangsu Province (17KJA620002), the Priority Academic Program Development of the Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, J., Yu, AD., Suetor, C.G. et al. Risk assessment of polyarylether polymerization process. J Therm Anal Calorim 144, 295–303 (2021). https://doi.org/10.1007/s10973-020-10084-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-10084-4