Abstract
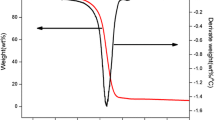
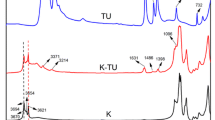
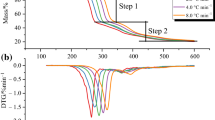
To analyze the feasibility of phosphorus-containing ionic liquids used as flame retardants on flammable materials, thermal stability and pyrolysis kinetics of 1-butyl-3-methylimdazolium dibutyl phosphate ([Bmim][DBP]) were investigated using nonisothermal thermogravimetry. The apparent onset decomposition temperature (T0) and mass fraction of residual carbon were 275.2–297.3 °C (± 0.5 °C) and 8.6–10.2% (± 0.1%), respectively. The apparent activation energy (Ea), pre-exponential factor (A), and most probable kinetic function [G(α)] were calculated using thermokinetic methods as Ea = 152–164 kJ mol−1 (± 2 kJ mol−1), ln A = 27.7 ± 0.4 s−1, and G(α) = − ln(1 − α). The maximum operation temperature was estimated as 166.0 ± 0.2 °C, which was considerably lower than T0. The pyrolysis products were identified through gas chromatograph/mass and Fourier transform infrared spectrometers. As a novel finding, the main flame-retarding mechanism of [Bmim][DBP] occurred primarily in condensed phase. Complementally, [Bmim][DBP] was testified to have the flame-retardant effect on epoxy resin by limited oxygen index and vertical burning tests.







Similar content being viewed by others
Abbreviations
- A :
-
Pre-exponential factor (s−1)
- α :
-
Fraction of conversion (mass%)
- β :
-
Heating rate (K min−1)
- C :
-
Constant
- C 0.25 :
-
Reaction order, n = 0.25
- C 1 :
-
First-order reaction
- C 3 :
-
Reaction order, n = 3
- dα/dt :
-
Mass loss rate (mass% min−1)
- (dα/dt)0.5 :
-
Mass loss rate at the conversion of 0.5 (mass% min−1)
- D 2 :
-
Valensi reaction
- D 3 :
-
Jander reaction
- 3D:
-
Z–L–T reaction
- E a :
-
Apparent activation energy (kJ mol−1)
- f(α):
-
Most probable kinetic function
- G(α):
-
Integral mechanism function
- [Him]+ :
-
1 H-imidazole
- ln A :
-
Logarithmic pre-exponential factor (s−1)
- m :
-
Fraction of mass residual (mass%)
- m/z :
-
Mass charge ratio (°C)
- [MHim]+ :
-
3-Methyl-1 H-imidazole
- [Mim]+ :
-
Methyl imidazole
- MOT:
-
Maximum operation temperature (°C)
- MOT1.0% :
-
Mass loss less than 1.0% of MOT (°C)
- R :
-
Universal gas constant (8.314 J mol−1 K−1)
- R 2 :
-
Regression coefficient
- t :
-
Time of reaction (min)
- T :
-
Temperature (°C)
- T 0.5 :
-
Temperature at the conversion of 0.5 (°C)
- T ed :
-
End temperature (°C)
- T m :
-
Maximum temperature (°C)
- T 0 :
-
Apparent onset decomposition temperature (°C)
- T p :
-
Peak temperature (°C)
- y 1(α):
-
Standard curve
- y 2(α):
-
Experimental curve
References
Maton C, Vos N, Stevens CV. Ionic liquid thermal stability: decomposition mechanism and analysis tools. Chem Soc Rev. 2013;42:5963–77.
Zheng L, Bu XX, Fan BH, Wei J, Xing NN, Guan W. Study on thermodynamic property for ionic liquid [C4mim][Lact] (1-butyl-3-methylimidazolium lactic acid). J Therm Anal Calorim. 2016;123:1619–25.
Kroon MC, Buijs W, Peters CJ, Witkamp GJ. Quantum chemical aided prediction of the thermal decomposition mechanism and temperature of ionic liquids. Thermochim Acta. 2007;465:40–7.
Tang G, Deng D, Chen J, Zhou KQ, Zhang H, Huang XJ, Zhou ZJ. The influence of organo-modified sepiolite on the flame-retardant and thermal properties of intumescent flame-retardant polylactide composites. J Therm Anal Calorim. 2017;130:763–72.
Tang S, Qian LJ, Liu XX, Dong YP. Gas-phase flame-retardant effects of a bi-group compound based on phosphaphenanthrene and triazine-trione groups in epoxy resin. Polym Degrad Stab. 2016;133:350–7.
Zhang L, Wang YC, Liu Q, Cai XF. Synergistic effects between silicon-containing flame retardant and DOPO on flame retardancy of epoxy resins. J Therm Anal Calorim. 2016;123:1343–50.
Wang YH, Zhang S, Wu XM, Lu CL, Cai YQ, Ma LJ, Shi G, Yang LT. Effect of montmorillonite on the flame-resistant and mechanical properties of intumescent flame-retardant poly(butylene succinate) composites. J Therm Anal Calorim. 2017;128:1417–27.
Xu JZ, He ZM, Wu WH, Ma HY, Xie JX, Qu HQ, Jiao YH. Study of thermal properties of flame retardant epoxy resin treated with hexakis[p-(hydroxymethyl)phenoxy]cyclotriphosphazene. J Chem Anal Calorim. 2013;114:1341–50.
Chen SJ, Wang CL, Li J. Effect of alkyl groups in organic part of polyoxometalates based ionic liquids on properties of flame retardant polypropylene. Thermochim Acta. 2016;631:51–8.
Yang XF, Ge NL, Hu LY, Gui HG, Wang ZG, Ding YS. Synthesis of a novel ionic liquid containing phosphorus and its application in intumescent flame retardant polypropylene system. Polym Adv Technol. 2013;24:568–75.
Chen SJ, Li J, Zhu YK, Guo ZB, Su SP. Increasing the efficiency of intumescent flame retardant polypropylene catalyzed by polyoxometalate based ionic liquid. J Mater Chem A. 2013;48:15242–6.
Sorai M. Comprehensive handbook of calorimetry and thermal analysis. Chichester: Wiley; 2004.
Ullah Z, Bustam MA, Man Z, Khan AS. Thermal stability and kinetic study of benzimidazolium based ionic liquid. Procedia Eng. 2016;148:215–22.
Kamavaram V, Reddy RG. Thermal stabilities of di-alkylimidazolium chloride ionic liquids. Int J Therm Sci. 2007;47:773–7.
Muhammad A. Thermal and kinetic analysis of pure and contaminated ionic liquid: 1-butyl-2, 3-dimethylimidazolium chloride (BDMIMCl). Pol J Chem Technol. 2016;18:122–5.
Heym F, Etzold BJM, Kern C, Jess A. An improved method to measure the rate of vaporization and thermal decomposition of high boiling organic and ionic liquids by thermogravimetrical analysis. Phys Chem Chem Phys. 2010;12:12089–100.
Abusaidi H, Ghaieni HR. Thermal analysis and kinetic decomposition of nitro-functional hydroxyl-terminated polybutadiene bonded explosive. J Chem Anal Calorim. 2017;127:2301–6.
Golofit T, Zielenkiewicz T. The influence of substituents position on products of dinitrotoluene isomers initial thermal decomposition. J Chem Anal Calorim. 2017;128:311–7.
Chen Y, Cao YY, Shi Y, Xue ZM, Mu TC. Quantitative research on the vaporization and decomposition of [EMIM][Tf2N] by thermogravimetric analysis–mass spectrometry. Ind Eng Chem Res. 2012;51:7418–27.
Seeberger A, Andresen AK, Jess A. Prediction of long-term stability of ionic liquids at elevated temperatures by means of non-isothermal thermogravimetrical analysis. Phys Chem Chem Phys. 2009;11:9375–81.
Navarro P, Larriba M, Rojo E, Garcia J, Rodríguez F. Thermal properties of cyano-based ionic liquid. J Chem Eng Data. 2013;58:2187–93.
Efimova A, Pfützner L, Schmidt P. Thermal stability and decomposition mechanism of 1-ethyl-3-methylimidazolium halides. Thermochim Acta. 2015;604:129–36.
Mohamed MA, Attia AK. Thermal behavior and decomposition kinetics of cinnarizine under isothermal and non-isothermal conditions. J Chem Anal Calorim. 2017;127:1751–6.
Sun DC, Yao YW. Synthesis of three novel phosphorus-containing flame retardants and their application in epoxy resins. Polym Degrad Stab. 2011;96:1720–4.
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Vyazovkin S, Chrissafis K, Lorenzo ML, Koga N, Pijolat M, Roduit B, Sbirrazzuoli N, Suñol JJ. ICTAC Kinetics Committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochim Acta. 2014;590:1–23.
Starink MJ. The determination of activation energy from linear heating rate experiments: a comparison of the accuracy of isoconversion methods. Thermochim Acta. 2003;404:163–76.
Friedman HL. Kinetics of thermal degradation of char-forming plastics from thermogravimetry: application to a phenolic plastic. J Polym Sci Part C. 1964;6:183–95.
Coats AW, Redfern JP. Kinetic parameters from thermogravimetric data. Nature. 1964;201:68–9.
Málek J, Smrčka V. The kinetic analysis of the crystallization processes in glasses. Thermochim Acta. 1991;186:153–69.
Málek J. A computer program for kinetic analysis of non-isothermal thermos-analytical data. Thermochim Acta. 1989;138:337–46.
Muhammad A, Mutalib AMI, Wilfred CD, Murugesan T, Shafeeq A. Thermophysical properties of 1-hexyl-3-methyl imidazolium based ionic liquids with tetrafluoroborate, hexafluorophosphate and bis(trifluoromethylsulfonyl)imide anions. J Chem Therm. 2008;40:1433–8.
Khan AS, Man Z, Bustam MA, Kait CF, Ullah Z, Nasrullah A, Khan MI, Gonfa G, Ahmad P, Muhammad N. Kinetics and thermodynamic parameters of ionic pretreated rubber wood biomass. J Mol Liq. 2016;223:754–62.
Karolina K, Beata G, Grzegorz G, Artur B, Zaneta KK. Thermal decomposition of binder based on etherified starch to use in foundry industry. J Therm Anal Calorim. 2017;130:285–90.
Parajó JJ, Teijeira T, Fernández J, Salgado J, Villanueva M. Thermal stability of some imidazolium [NTf2] ionic liquid: isothermal and dynamic kinetic study through thermogravimetric procedures. J Chem Thermodyn. 2017;112:105–13.
Salgado J, Villanueva M, Parajó JJ, Fernández J. Long-term thermal stability of five imidazolium ionic liquids. J Chem Thermodyn. 2013;65:184–90.
Shen MY, Kuan CF, Kuan SC, Chen CH, Wang JH, Yip MC, Chiang CL. Preparation, characterization, thermal, and flame-retardant properties of green silicon-containing epoxy/functionalized graphene nanosheets composites. J Nanomater. 2013;2013:363–71.
Acknowledgements
This study was very grateful to be supported by the Process Safety and Disaster Prevention Laboratory, Postgraduate Research and Practice Innovation Program of Jiangsu Province (KYCX17_0915). The authors appreciate the original suggestions and heartfelt inspiration for provided by the members of IL research groups.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jiang, HC., Lin, WC., Hua, M. et al. Analysis of thermal stability and pyrolysis kinetic of dibutyl phosphate-based ionic liquid through thermogravimetry, gas chromatography/mass spectrometry, and Fourier transform infrared spectrometry. J Therm Anal Calorim 138, 489–499 (2019). https://doi.org/10.1007/s10973-019-08229-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08229-1