Abstract
The aim of this study was the synthesis of three different epoxy compounds based on naphthalene-2,7-diol (2,7-NAF.EP, 2,7-NAF.WEP, 2,7-NAF.P.EP) and then their cross-linking by triethylenetetramine (TETA). All epoxides were prepared by the reaction of naphthalene-2,7-diol with epichlorohydrin but under different conditions and with other catalysts. The structures of the obtained compounds before and after the cross-linking reactions were confirmed by the attenuated total reflectance Fourier transform infrared spectroscopy (ATR/FT-IR). The ATR/FT-IR spectra of cross-linked compounds show disappearance of the C–O–C bands (about 915 cm−1) derived from the epoxy groups. DSC and TG/DTG measurements indicated that the obtained materials possess good thermal resistance; they are stable up to about 250 °C. The hardness of the cross-linked products was determined using the Shore D method. The highest value of hardness was obtained for the 2,7-NAF.EP-POL. Additionally, the UV–Vis absorption spectra of the obtained polymers were registered and evaluated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epoxy resins are very important class of thermosetting polymers which were synthesized by Prishajew in 1909 [1]. Chemical structures of the most important epoxy resins contain aliphatic, aromatic or cycloaliphatic groups and more than one epoxy group [2, 3]. These compounds found a wide range of applications in protective coatings, electronic-packaging materials, adhesives and high-performance composites, etc., due to good mechanical strength, strong adhesion, high moisture and solvent resistances, outstanding chemical resistance, good thermal and dimensional stabilities, superior electrical properties and wide formulation diversity [4,5,6,7,8,9,10,11,12,13,14,15]. However, the inherent brittle nature and poor crack resistance limited their applications significantly. Recently, much research has been carried out to toughen epoxy resins through the addition of flexible curing agents [16,17,18,19]. Wang et al. [19] prepared novel amines with different lengths of flexible polyoxypropylene side chains (AFPE). They used the obtained compound as a curing agent for diglycidyl ether of bisphenol A (DGEBA). DSC showed that the activation energy (Ea) value of DGEBA/AFPE increased from 45.2 to 52.4 kJ mol−1 with the increasing molecular weight of AFPE. Furthermore, when the AFPE was added into the epoxy networks, the impact strength and elongation at break improved significantly, which was mainly due to flexible polyoxypropylene side chains in the networks, decreasing cross-link density and increasing size of the cavities. Their study showed that AFPE was a novel and effective toughening agent for epoxy resins.
In general, uncured epoxy resins have only poor mechanical, chemical and heat resistance properties. However, good properties are obtained by reacting the linear epoxy resin with suitable hardeners to form three-dimensional cross-linked thermoset structures. Among commonly used curing agents for epoxy resins, amine-based ones, especially aromatic and aliphatic amines, are of prime significance in practical applications. Nowadays, most related scientific work is focused on aromatic-amine curing agents. Yet little attention is paid to developing aliphatic amine ones. Although aromatic-amine curing agents can endow their cured epoxy resins with improved thermomechanical properties and fire resistance, they still suffer from their low reactivity and high melting temperatures. For this reason, high-temperature cure must be applied to improve the compatibility of aromatic-amine curing agents with epoxy resins and to accelerate curing reactions. On the other hand, aliphatic-amine curing agents can cure epoxy resins efficiently at room temperature and at even somewhat lower temperatures without heating because they are characterized by high reactivity and low melting temperatures, accounting for their principal applications in room-temperature-cure epoxy coatings and adhesives [20,21,22,23,24,25,26].
Numerous studies on improvement of heat resistance of epoxy resins by increasing the cross-linking density of cured epoxy resin [27] or introducing bulky structures such as biphenyl or naphthalene [28, 29] were reported. The addition of naphthalene moiety is expected to improve greatly thermal property and moisture resistance [30,31,32]. Pan et al. [32] synthesized a series of novel novolac epoxy resins containing naphthalene moiety with different molecular weights via condensation of bisphenol A and 1-naphthaldehyde, followed by epoxidation with epichlorohydrin. They demonstrated that the cured naphthalene epoxy resin exhibited remarkably higher glass transition temperatures (Tg), enhanced thermal stability and better moisture resistance than the diglycidyl ether of bisphenol A (DGEBA). These pronounced good properties make it an attractive candidate for electronic encapsulation applications and composite materials. Xu et al. [33] conducted the synthesis of novel epoxy resin bearing naphthyl and limonene moieties. They showed that the obtained compounds have remarkably higher Tg, lower coefficient of thermal expansion, higher thermal stability, better moisture resistance and dielectric property.
This paper presents the synthesis of epoxy resins derivatives of naphthalene-2,7-diol: 2,7-NAF.EP, 2,7-NAF.P.EP and 2,7-NAF.WEP. These compounds were cross-linked using triethylenetetramine (TETA). The chemical structures of all new compounds were confirmed by spectroscopic methods. Thermal and luminescent properties of the obtained epoxy resins were also studied.
Experimental
Materials
Naphthalene-2,7-diol was purchased from Sigma-Aldrich (Germany). Epichlorohydrin was from Fluka (Switzerland). 2-Propanol, sodium hydroxide, toluene, propyl carbonate, potassium carbonate, potassium acetate, triethylenetetramine (TETA), acetic acid, butanol and xylene were obtained from Avantor Performance Materials Poland S.A. All above-mentioned chemicals were used as received.
Characterization of the products
The epoxide value (EV) was determined according to the following procedure. About 0.5 g of the tested resin was weighed on an analytical balance with an accuracy of 0.0001 g by introducing it into an Erlenmayer flask (200 mL). The resin was mixed with 13 mL of the hydrochloric acid solution in dioxane. Then, five drops of cresol red solution were introduced into this mixture and were titrated with 0.2 M alcohol solution of NaOH. During neutralization, the color of the solution changed from red to purple via yellow.
The epoxide value is determined according to the formula:
where V1—the amount of 0.2 M NaOH used for the titration of the blank test [mL], V2—the amount of 0.2 M NaOH used for the titration of the test sample [mL], n—concentration of NaOH standard aqueous solution (mol L−1) and m—mass of the sample (g). The epoxide value (EV) is the number of epoxide equivalents in 100 g of resin (eq./100 g) as used in this study.
Attenuated total reflection-Fourier transform infrared (ATR/FT-IR) spectra were recorded on a Bruker FTIR spectrophotometer TENSOR 27 (Bruker, Germany) using thin films of epoxy compounds. Spectra were gathered from 4000 to 600 cm−1 averaging 32 scans with a resolution of 4 cm−1.
Differential scanning calorimetry (DSC) curves were obtained on a DSC Netzsch 204 calorimeter (Netzsch, Günzbung, Germany). DSC measurements were taken in the aluminium pans with a pierced lid of the sample weight of ~ 5 to 15 mg in the nitrogen atmosphere (30 mL min−1). The empty aluminium crucible was applied as a reference. Dynamic scans were obtained at a heating rate of 10 K min−1 in the temperature range 20–550 °C. The parameters such as: decomposition temperatures (Tonset, Toffset), final decomposition temperature (Td) and enthalpy of decomposition (ΔHd) were determined.
Thermogravimetric analysis (TG/DTG) was made with the use of a thermal analyzer STA 449 F1 Jupiter (Netzsch, Selb, Germany) with the sample mass of ~ 5 to 10 mg in the helium atmosphere (20 mL min−1). Dynamic scans were made at the heating rate of 10 °C min−1 in the temperature range 0–600 °C. The main parameters of the thermal degradation were as follows: TIDT—the initial decomposition temperature of the sample, T20—the temperature at 20% mass loss, T50—the temperature for 50% mass loss, peak maximum decomposition temperature (Tmax), mk—the final mass loss at 600 °C, Tf—the final decomposition temperature. All measurements were taken in the Al2O3 crucible. As a reference, the empty Al2O3 crucible was used.
The hardness of the epoxy compounds was measured by the Shore D method on a Zwick 7206/H04 hardness tester (Germany) at 298 K. The values were taken after 15 s.
Room temperature UV–Vis reflectance spectra were registered using a horizontal sampling integrating sphere (Model PIV-756) connected to a V-660 JASCO spectrophotometer.
Synthesis of epoxy compounds
Synthesis of epoxy resin of 2,7-NAF.EP
The synthesis of the epoxy resin was performed in a 750-mL round-bottom flask equipped with a mechanical stirrer, a thermometer and a dropping funnel. 195 mL of epichlorohydrin and 141.66 mL of 2-propanol were poured into the flask, and then, 40 g of naphthalene-2,7-diol was added. The mixture was heated to a temperature of about 70 °C. Subsequently, 22.5 mL of a 15% NaOH solution was added dropwise at this temperature and heating was continued for 30 min. The sodium hydroxide solution closes the epoxy cycle by consuming the resulting hydrochloric acid. After cooling, the solution was poured into a separating funnel, the inorganic layer was poured off, and isopropanol and unreacted epichlorohydrin were distilled from the upper organic layer. Then, 30 mL of toluene was added to the flask and distilled again. After removing the by-products, an epoxy resin (2,7-NAF.EP) with the epoxy number 0.62 (n = 0) was prepared. The scheme of the obtained resin synthesis is shown in Fig. 1 [34, 35].
Synthesis of epoxy resin with an average molecular weight of derivative naphthalene-2,7-diol (2,7-NAF.WEP)
In a 250-mL three-necked flask equipped with a thermometer, a dropping funnel and a Teflon mixer, there were placed 16 g (0.1 mol) of naphthalene-2,7-diol, 18.5 g (0.2 mol) of epichlorohydrin and 10 mL of toluene. The contents in the flask were heated to 90 °C. Then, 60 mL of a 15% aqueous NaOH solution was added dropwise over 2 h. After the NaOH addition, the reaction was continued for 2 h keeping the temperature at 90 °C. After cooling, 10 mL of 20% CH3COOH, 20 mL of butanol and 20 mL of toluene were added to the reaction mixture. Next after heating to 70 °C, the contents of the flask were poured into a separating funnel and the aqueous layer was separated. The organic layer was placed in a flask from which the solvents and residual H2O were distilled off under vacuum [35]. After separation of the reaction by-products, an epoxy resin (2,7-NAF.WEP) having the epoxide number 0.28 was obtained. The synthesis is presented in Fig. 2.
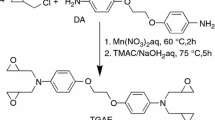
Synthesis of 2,7-di[2-(2,3-epoxypropoxy)propoxy]naphthalene (2,7-NAF.P.EP)
The synthesis of 2,7-di[2-(2,3-epoxypropoxy)propoxy]naphthalene proceeded in two stages which are presented in Figs. 3 and 4 [36].
In the first stage, 43 g of naphthalene-2,7-diol, 55.1 g of propyl carbonate and 0.13 g of potassium carbonate as a catalyst were added to a round-bottom flask of 250 mL equipped with a thermometer, mechanical stirrer, reflux condenser and a pipe for introducing liquid nitrogen. The reaction was carried out at high temperature in the range of 210–220 °C. During the 2.5 h reaction, the release of CO2 was evident. When the gas ceased to be released, the reaction was continued for 0.5 h. Then, 200 mL of chloroform and 150 mL of distilled water were added to the flask. The contents of the flask were transferred to a separating funnel. After careful separation of the phases, the solvents were distilled from the organic layer. As a result, 2,7-di(2-hydroxypropoxy)naphthalene was obtained.
In the second stage, the epoxidation reaction was performed using epichlorohydrin. The molar ratio of epichlorohydrin to the resulting diol was 10. 51 g of 2,7-di[2-hydroxypropoxy]naphthalene; 150 mL of epichlorohydrin and 0.4 g of anhydrous potassium acetate were placed in the 500-mL round-bottom flask which was equipped with a thermometer, mechanical stirrer and the Dean–Stark trap for azeotropic water separation. The reaction mixture was heated to 90 °C until all reactants were dissolved. Next, 18.5 g of NaOH suspended in 70 mL of xylene was added to the flask. During this reaction, NaCl precipitated and the resulting water was azeotropically distilled using the Dean–Stark trap. After the reaction, 400 mL of toluene was added to the flask. The whole content was heated for about 15 min. NaCl was separated, and the solvents were distilled from the filtrate. The epoxy resin (2,7-NAF.P-EP) was obtained in the form of a slightly yellow, viscous liquid with the epoxide number 0.32 [37].
Curing of epoxy compounds using triethylenetetramine (TETA)
Curing (cross-linking) of epoxy resins takes place in the presence of suitable amines, anhydrides, organic acids or phenols. In this case, amine (triethylenetetramine) was used as a hardener. TETA (triethylenetetramine) was applied to convert the resulting epoxy compounds into infusible and insoluble products [38]. The polyaddition reaction of amines to epoxy resins is a complicated process in which the amine reacts with the epoxide oxygen and forms a hydroxyl group (Fig. 5):
The amount of amine for complete curing of 100 g epoxy resin is calculated from the formula:
where m—the mass of amine (g), EV—the epoxy number (mol/100 g), M—the molar mass of epoxy resin (g mol−1) and n—the number of active hydrogens.
The obtained epoxy resins (2,7-NAF.EP, 2,7-NAF.WEP, 2,7-NAF.P.EP) were weighed in suitable polyethylene containers. Next, a specific amount of TETA was added to each container and mixed thoroughly. The amount of TETA for individually weighed epoxy compounds was calculated from the proportions presented in Table 1.
The curing process was conducted at room temperature for 20 h. Then, the process was continued at 80 °C for 4 h. As a result of epoxy resins curing, solid products exhibiting photoluminescent properties were obtained (Fig. 6).
Results and discussion
ATR/FT-IR analysis
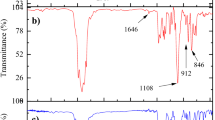
Figure 7 shows the ATR/FT-IR spectra of the epoxide derivative of naphthalene-2,7-diol: 2,7-NAF.EP and its cross-linked product: 2,7-NAF.EP-POL. In the 2,7-NAF.EP spectrum, the presence of main peaks located at 3467 and 912 cm−1 can be assigned to the O–H bands and the C–O deformation band from the epoxy group. C–H stretching of the terminal oxirane group is observed at 3030 cm−1. Also the signals located at 2930–3002 cm−1 are derived from the stretching vibrations of aromatic and aliphatic –CH, –CH2 groups. The aromatic group C=C gives a shape signal at 1627 cm−1. The peaks situated at 1210 and 1135 cm−1 are characteristic of C–O–C of the ester-type bonds. The signal at 832 cm−1 can be assigned to the C–H vibrations from the benzene ring.
In turn, in the spectrum of 2,7-NAF.EP-POL, the stretching vibrations of the hydroxyl group at 3332 cm−1 were observed. The group C=C gives a signal at 1626 cm−1. The signals derived from the aromatic C–H group are present at the wavelength of about 830 cm−1. The visible absorption band at the wavelength of about 1120 cm−1 indicates the vibration of C–O–C bonds. The spectrum also shows disappearance of the C–O bands derived from the epoxy group which indicates the proper course of curing reactions. Additionally, the stretching vibration of C–N group occurs at 1170 cm−1 and also the N–H bending signal is visible at 1490 cm−1.
Figures 8 and 9 show the ATR/FT-IR spectra of 2,7-NAF.P.EP; 2,7-NAF.WEP and their hardened products: 2,7-NAF.P.EP-POL and 2,7-NAF.WEP-POL. The spectra of 2,7-NAF.WEP; 2,7-NAF.P.EP and their hardened products present the same type of signals located at approximately the same wavelengths as in the case of the spectra for 2,7-NAF.EP and 2,7-NAF.EP-POL.
DSC analysis
The DSC curves of the compounds: 2,7-NAF.EP; 2,7-NAF.WEP, and 2,7-NAF.P.EP and their cured products (2,7-NAF.EP-POL; 2,7-NAF.WEP-POL; 2,7-NAF.P.EP-POL) are shown in Figs. 10–12. In addition, the characteristic temperatures: Tonset, Toffset, Td and the enthalpy of decomposition (ΔHd) are given in Table 2.
The DSC analysis showed the differences in thermal behaviour of the obtained epoxy resins (2,7-NAF.EP; 2,7-NAF.P.EP, and 2,7-NAF.WEP) and their cross-linked polymers. The DSC curves of 2,7-NAF.EP and its polymer (Fig. 10) show one distinct endothermic peak. The endothermic peaks at 333–345 °C with the ΔHd values from 85 to 256 J g−1 correspond with the total thermal degradation of these compounds.
The DSC curves of the other compounds: 2,7-NAF.P.EP and its polymer show also one endothermic peak (Fig. 11). This peak occurs in the temperature range at 341–368 °C and may result the thermal degradation of 2,7-NAF.P.EP and 2,7-NAF.P.EP-POL. Moreover, the copolymers were characterized by rather high thermal stability, and no endothermic decomposition peak up to 250 °C was observed.
In the case of DSC curves of 2,7-NAF.WEP and its polymer (Fig. 12), two endothermic peaks were found. Probably, the determined DSC curves are related to the macromolecular structures of the resulting copolymer. The first endothermic peaks at 329–349 °C with the ΔHd values from 21 to 35 J g−1 demonstrated the degradation of low cross-linked, linear fragments of copolymer. The second effect (at 421–423 °C) is associated with the thermal degradation of aromatic fragments of 2,7-NAF.WEP and its polymer.
TG/DTG study
Thermal stabilities and degradation behaviour of the cross-linked resins were studied by means of thermogravimetry in the helium atmosphere. The curves obtained from the TG and DTG measurements are shown in Fig. 13. The obtained data are also summarized in Table 3. Analyzing the obtained data, one can conclude that the largest thermal resistance was found in the case of 2,7-NAF.EP-POL. This material decomposed in one main step in the range 291–424 °C with the maximum at 350 °C. The rate of the mass loss for 2,7-NAF.EP is − 11.07% min−1. For the 2,7-NAF.P.EP-POL sample, the decomposition process proceeds from 217 to 427 °C with the maximum at 365 °C. This compound decomposes with the rate of − 11.1% min−1. In the case of 2,7-NAF.WEP-POL, the thermal decomposition proceeds with the slowest rate (− 8.85% min−1). For this sample, the maximum of the decomposition process is bimodal and takes place at 366 and 410 °C, respectively. On the DTG curve for the 2,7-NAF.WEP-POL also one small decomposition stage can be noted. The peak maximum is observed at 180 °C and is probably connected with degradation of the low-cross-linked linear, aliphatic fragments of resins. The final decomposition temperatures (Tf) are from 424 to 430 °C.
For overall assessment of thermal resistance of the obtained resins, the analysis in air atmosphere was also carried out. Figure 14 shows TG/DTG curves; additionally, the obtained data are collected in Table 4. Slight decrease (5–25 °C) of the initial decomposition temperature was noted for all samples compared with analysis in inert conditions. Initial decomposition temperature (IDT) for the 2,7-NAF.EP-POL was 305 °C, while the final decomposition temperature was 446 °C. This sample was degraded in single step (maximum in 345 °C). The main decomposition range for 2,7-NAF.WEP-POL resin was from 300 °C to 464 °C. On DTG curve for this material, one small decomposition stage at 188 °C was noticeable (similar like in helium atmosphere). Two maxima of decomposition process were observed for this sample: first at 355 °C and second at 424 °C. Decomposition process for 2,7-NAF.P.EP-POL proceeded in one main step with maximum rate in temperature 350 °C. The range of degradation for 2,7-NAF.P.EP-POL was from 230 to 442 °C.
The type of atmosphere in which thermal decomposition was carried out also affects the speed of this decomposition. For example, material 2,7-NAF.EP-POL decomposes in helium with the rate of − 11.07% min−1 but in air atmosphere the maximum rate of the mass loss was − 8.18% min−1. In helium, the rate of the mass loss for 2,7-NAF.WEP-POL for first maximum peak is − 6.75% min−1, and for second maximum is − 8.85% min−1 while in case distribution in air the first maximum of decomposition is − 5.49% min−1 and the second is − 4.85% min−1. In the case of 2,7-NAF.P.EP-POL, the maximum rate of thermal decomposition in helium atmosphere is − 11.1% min−1 while in air conditions this value is − 4.73% min−1.
The shape of TG and DTG curves received in air conditions is close of the shape these curves obtained in helium atmosphere. The synthesized epoxy resins generally are characterized by good thermal resistance and stability.
The study of epoxy compounds hardness
Hardness of plastics is defined as the resistance of a material while pressing a vertically positioned indenter into its surface. The pressure of the indenter must be so large as to make the permanent deformation. The greater the hardness of the material, the smaller the depth of the dip, and thus the greater the load on the indenter. The principle of measurement is based on measuring the depth of indenter penetration into the material of the studied polymer samples on a scale from 0 to 100°Sh. The results of the study are shown in Table 5. The highest value of hardness was characterized by 2,7-NAF.EP-POL, while the lowest value was assigned to 2,7-NAF.P.EP-POL. These results are a consequence of chemical structure of the epoxides. The lowest values were obtained for the resins possessing the longest aliphatic chain.
Absorbance measurements of the obtained epoxy resins
The studied epoxides exhibit a strong UV absorption at approximately 350 nm, associated with the presence of the naphthalene ring. In the absorption spectra (Fig. 15), peaks are also visible at about 450 nm for 2,7-NAF.EP-POL; 2,7-NAF.WEP-POL, and 2,7-NAF.P.EP-POL related to the presence of epoxide rings in the -2,7- position in naphthalene. The highest value of absorbance was found for 2,7-NAF.EP-POL, and the lowest value for 2,7-NAF.P.EP-POL. After excitation, both the epoxides and their polymers emitted yellow-green radiation [36, 37].
Conclusions
The synthesis and characterization of new cross-linked polymeric naphthalene derivatives with unique luminescent properties are presented. As a result of the reactions of naphthalene-2,7-diol with epichlorohydrin, three epoxide compounds were obtained under various conditions and using other catalysts. All obtained epoxy derivatives undergo cross-linking under the influence of triethylenetetramine giving solid products with different hardness.
These materials are characterized by good thermal resistance. The final decomposition temperatures for all compositions are from 424 to 430 °C. After excitation of UV radiation, the copolymers (and epoxides) emitted yellow-green radiation. This property makes the naphthalene derivatives very useful precursors for preparation of e.g.: fluorophores of luminescent materials, polymer light-emitting diodes and organic semiconductors. Easily processable fluorescent polymers containing naphthol chromophores could have potential applications in the production of high laser-resistant materials, laser dyes or fiber optic sensors. Due to suitable thermal resistance, the obtained epoxy resins can be used in the production of thermally resistant coatings.
References
May CA. Epoxy resins, chemistry and technology. 2nd ed. New York: Marcel Dekker; 1988.
Mustata F, Tudorachi N. Curing kinetics and thermal characterization of epoxy resin cured with amidodicarboxylic acids. Appl Therm Eng. 2017;125:285–96.
Pascault JP, Williams RJJ. Epoxy polymers—new materials and innovations. Weinheim: Wiley; 2010.
Fraga F, Soto V, Rodríguez-Núñez E, Martínez-Ageitos J, Rodríguez V. Cure kinetic of the epoxy network diglycidyl ether of bisphenol A (BADGE n = 10)/amantidine. J Therm Anal Calorim. 2007;87:97–100.
Guo Q, Huang Y, Zhang YY, Zhu LR, Zhang BL. Curing behavior of epoxy resins with a series of novel curing agents containing 4,4′-biphenyl and varying methylene units. J Therm Anal Calorim. 2010;102:915–22.
Ghaemy M, Behmadi H. Study of cure kinetics of DGEBA with optically active curing agents. J Therm Anal Calorim. 2010;101:1011–7.
Zhang D, Jia D, Chen S. Kinetics of curing and thermal degradation of hyperbranched epoxy (HTDE)/diglycidyl ether of bisphenol-A epoxy hybrid resin. J Therm Anal Calorim. 2009;98:819–24.
Jin H, Miller GM, Pety SJ, Griffin AS, Stradley DS, Roach D, Sottos NR, White SR. Fracture behavior of a self-healing, toughened epoxy adhesive. Int J Adhes Adhes. 2013;44:157–65.
Kaboorani A, Riedl B. Nano-aluminum oxide as a reinforcing material for thermoplastic adhesives. J Ind Eng Chem. 2012;18:1076–81.
Azeez AA, Rhee KY, Park SJ, Hui D. Epoxy clay nanocomposites—processing, properties and applications: a review. Compos Part B. 2013;45:308–20.
Conradi M, Kocijan A, Kek-Merl D, Zorko M, Verpoest I. Mechanical and anticorrosion properties of nanosilica-filled epoxy-resin composite coatings. Appl Surf Sci. 2014;292:432–7.
Katariya MN, Jana AK, Parikh PA. Corrosion inhibition effectiveness of zeolite ZSM-5 coating on mild steel against various organic acids and its antimicrobial activity. J Ind Eng Chem. 2013;19:286–91.
Liu Q, Bao X, Deng SQ. The investigation of methyl phenyl silicone resin/epoxy resin using epoxy-polysiloxane as compatibilizer. J Therm Anal Calorim. 2014;118:247–54.
Zhang L, Wang Y, Liu Q, Cai X. Synergistic effects between silicon-containing flame retardant and DOPO on flame retardancy of epoxy resins. J Therm Anal Calorim. 2016;123:1343–50.
Luo H, Zhou F, Yang Y, Cao X, Cai X. Synergistic flame-retardant behavior and mechanism of tris(3-nitrophenyl) phosphine and DOPO in epoxy resins. J Therm Anal Calorim. 2018;132:483–91.
Yang G, Fu SY, Yang JP. Preparation and mechanical properties of modified epoxy resins with flexible diamines. Polymer. 2007;48:302–10.
Cai H, Li P, Sui G, Yu Y, Li G, Yang X, Ryu S. Curing kinetics study of epoxy resin/flexible amine toughness systems by dynamic and isothermal DSC. Thermochim Acta. 2008;473:101–5.
Park SJ, Jin FL, Park JH, Kim KS. Synthesis of a novel siloxane-containing diamine for increasing flexibility of epoxy resins. Mater Sci Eng. A. 2005;399:377–81.
Wang G, Jiang G, Zhang J. Preparation, curing kinetic and properties of a novel amine with flexible polyoxypropylene side chain curing agent for epoxy resin. Thermochim Acta. 2014;589:197–206.
Hakiki F, Salam DD, Akbari A, Nuraeni N, Aditya W, Siregar S. Is epoxy-based polymer suitable for water shut-off application? Soc Pet Eng. 2015. https://doi.org/10.2118/176457-MS.
Li C, Zuo C, Fan H, Yu M, Li B. Novel silicone aliphatic amine curing agent for epoxy resin: 1,3-Bis(2-aminoethylaminomethyl) tetramethyldisiloxane. 1. Non-isothermal cure and thermal decomposition property. Thermochim Acta. 2012;545:75–81.
Petrie EM. Epoxy adhesive formulations. New York: McGraw-Hill Publishing; 2006.
Perrin FX, Nguyen TMH, Vernet JL. Chemico-diffusion kinetics and TTT cure diagrams of DGEBA–DGEBF/amine resins cured with phenol catalysts. Eur Polym J. 2007;43:5107–20.
Li Y, Xiao F, Wong CP. Novel, environmentally friendly crosslinking system of an epoxy using an amino acid: tryptophan-cured diglycidyl ether of bisphenol A epoxy. J Polym Sci Part A Polym Chem. 2007;45:181–90.
Wan J, Li C, Bu ZY, Xu CJ, Li BG, Fan H. A comparative study of epoxy resin cured with a linear diamine and a branched polyamine. Chem Eng J. 2012;188:160–72.
Wan J, Bu ZY, Xu CJ, Li BG, Fan H. Preparation, curing kinetics, and properties of a novel low-volatile starlike aliphatic-polyamine curing agent for epoxy resins. Chem Eng J. 2011;171:357–67.
Wang CS, Lee MC. Synthesis, characterization, and properties of multifunctional naphthalene-containing epoxy resins cured with cyanate ester. J Appl Polym Sci. 1999;73(9):1611–22.
Matsumoto A, Hasegawa K, Fukuda A. Studies on modified phenolic resin. IV: properties of phenolic resin modified with 4-hydroxyphenylmaleimide/n-butylacrylate copolymers. Polym Int. 1993;30(1):65–72.
Ohta K, Kosaka W, Yanagisawa K. Eur Patent A 20,428,871 (1991).
Wang CS, Lee MC. Synthesis and modification of a naphthalene-containing trifunctional epoxy resin for electronic applications. J Appl Polym Sci. 1998;70(10):1907–21.
Yang CP, Chen WT. Synthesis and properties of new polyimides derived from 1,5-bis(4-aminophenoxy)naphthalene and aromatic tetracarboxylic dianhydrides. J Polym Sci Part A Polym Chem. 1993;31(11):2799–807.
Pan G, Du Z, Zhang C, Li C, Yang X, Li H. Synthesis, characterization, and properties of novel novolac epoxy resin containing naphthalene moiety. Polymer. 2007;48:3686–93.
Xu K, Chen MC, Zhang K, Hu JW. Synthesis and characterization of novel epoxy resin bearing naphthyl and limonene moieties, and its cured polymer. Polymer. 2004;45(4):1133–40.
Podkościelna B. New photoluminescent copolymers of naphthalene-2,7-diol dimethacrylate and N-vinyl-2-pyrrolidone: synthesis, characterisation and properties. J Therm Anal Calorim. 2014;116:785–93.
Bluestein C, Rosenblatt W, Clark J. Accelerated process for diaryl sulfone glicydyl ethers. US Patent 3790602 (1974).
Podkościelna B, Lipke A, Majdan M, Gawdzik B, Bartnicki A. Thermal and photoluminescence analysis of a diester of a methacrylic acid derivative of naphthalene-2,7-diol. J Therm Anal Calorim. 2016;126:161–70.
Podkościelna B. Method for preparing photoluminescent copolymer. Patent PL402734 (2015).
Podkościelna B, Lipke A, Gawdzik B, Majdan M. Synthesis, characterization and luminescent properties of new copolymers of dimethacrylate derivatives of naphthalene-2,7-diol. Polym Adv Technol. 2015;26:176–81.
Acknowledgements
The authors would like to thank Dr. Andrzej Bartnicki (UMCS Lublin) for his help in the synthesis of epoxy resins.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Fila, K., Gargol, M., Goliszek, M. et al. Synthesis of epoxy resins derivatives of naphthalene-2,7-diol and their cross-linked products. J Therm Anal Calorim 138, 4349–4358 (2019). https://doi.org/10.1007/s10973-019-08852-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08852-y