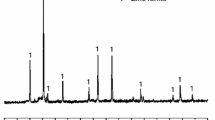
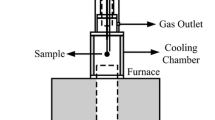
Abstract
Zinc ferrite is one of the main components present in the electric arc furnace dust. Furthermore, it is necessary to know the mechanisms involved on the zinc ferrite to determine a process for reusing the electric arc furnace dust. Thus, the aim of this paper is to evaluate the kinetic parameters of the reduction of synthetic zinc ferrite pellets by mixture containing hydrogen. The kinetic investigation was performed via Forced Stepwise Isothermal Analysis method in the temperature range of 500–950 °C. The reducing gas was a mixture of hydrogen (10%) and argon (90%). In addition, Thermo-cal software was used in order to support the reduction reaction behavior. The results indicated that between 550 and 750 °C only iron oxide was reduced. In this step, the nucleation was found to be the controlling mechanism, and an activation energy of 84.9 kJ mol−1 was determined. In temperature >800 °C, it was noted an influence of diffusion on the reduction reaction. Images obtained from scanning electron microscope suggested a change in the pellets microstructure after this temperature presenting a sintered structure. The activation energy of 188 kJ mol−1 was determined at this step.







Similar content being viewed by others
References
Wang HG, Gao JM, Liu W, Zhang M, Guo M. Recovery of metal-doped zinc ferrite from zinc-containing electric arc furnace dust: process development and examination of elemental migration. Hydrometallurgy. 2016;166:1–8.
Wu CC, Chang FC, Chen WS, Tsai MS, Wang YN. Reduction behavior of zinc ferrite in EAF-dust recycling with CO gas as a reducing agent. J Environ Manag. 2014;143:208–13.
Miki T, Fujimoto RC, Maruyama K, Nagasaka T. Hydrometallurgical extraction of zinc from CaO treated EAF dust in ammonium chloride solution. J Hazard Mater. 2016;302:90–6.
Nezhad SMM, Zabett A. Thermodynamic analysis of the carbothermic reduction of electric arc furnace dust in the presence of ferrosilicon. Calphad. 2016;52:143–51.
Fares G, Al-Zaid RZ, Fauzi A, Alhozaimy AM, Al-Negheimish AI, Khan MI. Performance of optimized electric arc furnace dust-based cementitious matrix compared to conventional supplementary cementitious materials. Constr Build Mater. 2016;112:210–21.
Vieira CMF, Sanchez R, Monteiro SN, Lalla N, Quaranta N. Recycling of electric arc furnace dust into red ceramic. J Mater Res Technol. 2013;2:88–92.
Alsheyaba MAT, Khedaywi TS. Effect of electric arc furnace dust (EAFD) on properties of asphalt cement mixture. Resour Conserv Recycl. 2013;70:38–43.
Suetens T, Klaasen B, Acker KV, Blanpain B. Comparison of electric arc furnace dust treatment technologies using exergy efficiency. J Clean Prod. 2014;65:152–67.
Menad N, Ayala JN, Carcedo FG, Hernández A. Study of the presence of fluorine in the recycled fractions during carbothermal treatment of EAF dust. Waste Manag. 2003;23:483–91.
Tong LF, Hayes P. Mechanisms of the reduction of zinc ferrites in H2/N2 gas mixtures. Miner Process Extract Metall Rev. 2007;28:127–57.
Junca E, Oliveira JR, Restivo TAG, Espinosa DCR, Tenório JAS. Synthetic zinc ferrite reduction by means of mixtures containing hydrogen and carbon monoxide. J Therm Anal Calorim. 2016;123:631–41.
Gioia F, Mura G, Viola A. Experimental study of the direct reduction of sinterized zinc oxide by hydrogen. Chem Eng Sci. 1977;32:1401–9.
Junca E, Restivo TAG, Oliveira JR, Espinosa DCR, Tenório JAS. Reduction of electric arc furnace dust pellets by simulated reformed natural gas. J Therm Anal Calorim. 2016;126:1889–97.
Viswanath RP, Viswanathan B, Sastri MVC. Kinetics and mechanism of reduction of ferric oxide by hydrogen. Trans JIM. 1977;18:149–54.
Lina HY, Chena YW, Li C. The mechanism of reduction of iron oxide by hydrogen. Thermochim Acta. 2003;400:61–7.
Junca E, Restivo TAG, Oliveira JR, Espinosa DCR, Tenório JAS. Application of stepwise isothermal analysis method in the kinetic study of reduction of basic oxygen furnace dust. J Therm Anal Calorim. 2015;120:1913–9.
Gretz J, Korf W, Lyons R. Hydrogen in the steel industry. J Hydrogen Energy. 1991;16:691–3.
Bahgat M, Khedr MH. Reduction kinetics, magnetic behavior and morphological changes during reduction of magnetite single crystal. Mater Sci Eng, B. 2007;138:251–8.
Tiernan MJ, Barnes PA, Parkes GMB. Reduction of iron oxide catalysts: the investigation of kinetic parameters using rate perturbation and linear heating thermo analytical techniques. J Phys Chem B. 2001;105:220–8.
Caceres PG, Behbehani MH. Microstructural and surface area development during; hydrogen reduction of magnetite. Appl Catal A: Gen. 1994;109:211–23.
Maqueda LAP, Ortega A, Criado JM. The use of master plots for discriminating the kinetic model of solid state reactions from a single constant-rate thermal analysis (CRTA) experiment. Thermochim Acta. 1996;277:165–73.
Chen F, Sorensen OT, Meng G, Peng D. Thermal decomposition of BaC2O4·0.5H2O studied by stepwise isothermal analysis and non-isothermal thermogravimetry. J Therm Anal. 1998;53:397–410.
Yu D, Zhu M, Utigard TA, Barati M. TG/DTA study on the carbon monoxide and graphite thermal reduction of a high-grade iron nickel oxide residue with the presence of siliceous gangue. Themochim Acta. 2014;575:1–11.
Yu D, Zhu M, Utigard TA, Barati M. TGA kinetic study on the hydrogen reduction of an iron nickel oxide. Miner Eng. 2013;54:32–8.
Rao YK. Mechanism and the intrinsic rate of reduction of metallic oxides. Metall Trans B. 1979;10:243–55.
Lin Q, Liu R, Chen N. Kinetics of direct reduction of chrome iron ore. J Therm Anal Calorim. 1999;58:317–22.
Weiss B, Sturn J, Voglsam S, Strobl S, Mali H, Winter F, Schenk J. Structural and morphological changes during reduction of hematite to magnetite and wustite in hydrogen rich reduction gases under fluidised bed conditions. Ironmak Steelmak. 2011;38:65–73.
Acknowledgements
The authors would like to thank FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) process 11/51638-0, the CNPq (Conselho Nacional de desenvolvimento Científico e Tecnológico), process 245470/2012-3, the CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), project PE003/2008, University of São Paulo and University of Extremo Sul Catarinense.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Junca, E., Grillo, F.F., Restivo, T.A.G. et al. Kinetic investigation of synthetic zinc ferrite reduction by hydrogen. J Therm Anal Calorim 129, 1215–1223 (2017). https://doi.org/10.1007/s10973-017-6222-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6222-7