Abstract
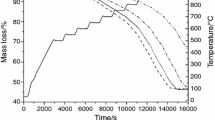
Basic oxygen furnace (BOF) dust is a by-product of steel plants that is rich in iron oxide. Reduction of this by-product may provide reutilization alternatives in steel industry. In this context, kinetic study was conducted using BOF dust pellets applying the forced stepwise isothermal analysis method. This work examines the reduction of BOF dust pellets using a mixture between hydrogen and carbon monoxide. Mass loss of 26 % was obtained after reduction of the BOF. Carbon oxide present in the reducing gas led to the formation of a carbon layer around the pellet between 550 and 650 °C. At higher temperatures, from 700 to 850 °C, it was observed that reduction was controlled by porous diffusion. In the range 900–1,000 °C, the control mechanism was determined as reactive species diffusion through metallic alloy lattice in individual particles.






Similar content being viewed by others
References
Szekely J. A research program for the minimization and effective utilization of steel plant wastes. Iron Steelmak. 1995;22:25–9.
Nyirenda RL. The processing of steelmaking flue-dust: a review. Miner Eng. 1991;7–11:1003–25.
Mikhail SA, Turcotte AM. Thermal reduction of steel-making secondary materials I. Basic-oxygen-furnace dust. Thermochim Acta. 1998;311:113–9.
Kelebek S, Yörük S, Davis B. Characterization of basic oxygen furnace dust and zinc removal by acid leaching. Miner Eng. 2004;17:285–91.
Evestedt M, Medvedev A. Model-based slopping warning in the LD steel converter process. J Process Control. 2009;19:1000–10.
Hay SM, Rankin WJ. Recovery of iron and zinc from blast furnace and basic oxygen furnace dusts: a thermodynamic evaluation. Miner Eng. 1994;7:985–1001.
Scheele JV, Johansson M. XYFINES a new technology for in-plant recycling of dust and sludge in metal production industries, Recycling and waste treatment in mineral and metal processing. Tech Econ Asp. 2002;1:109–18.
Makkonen HT, Heino J, Laitila L, Hiltunen A, Pöyliö E, Härkki J. Optimisation of steel plant recycling in Finland: dusts, scales and sludge. Resour Conserv Recycl. 2002;35:77–84.
Atkins P, De Paula J. Physical chemistry. 8th ed. New York: W. H. Freeman and Company; 2006.
Sorensen OT. Quasi-isothermal Methods in thermal analysis. Thermoch Acta. 1981;50:163–75.
Husum PL, Sorensen OT. Computer controlled forced stepwise isothermal analysis. Thermochim Acta. 1987;114:131–8.
Sorensen OT. Thermogravimetric and dilatometric studies using stepwise isothermal analysis and related techniques. J Therm Anal. 1992;38:213–28.
Gotor FJ, Maqueda LAP, Ortega A, Criado JM. Kinetic analysis of solid state reactions by means of stepwise analysis isothermal (SIA) and constant rate thermal analysis (CRTA). A comparative study. J Therm Anal Calorim. 1998;53:389–96.
Srinivasan NS. Reduction of iron oxides by carbon in a circulating fluidized bed reactor. Powder Technol. 2002;124:28–39.
Viswanath RP, Viswanathan B. Kinetics and mechanism of reduction of ferric oxides by hydrogen. Trans JIM. 1977;18:149–54.
Valipour MS, Hashemi MYM, Saboohi Y. Mathematical modeling of the reaction in an iron ore pellet using a mixture of hydrogen, water vapor, carbon monoxide and carbon dioxide: an isothermal study. Adv Powder Technol. 2006;17:277–95.
El-Geassy AA, Nasr MI, Hessien MM. Effect of reducing gas on the volume change during reduction of iron oxide compacts. ISIJ Int. 1996;36:640–9.
Pang JM, Guo PM, Zhao P, Cao CZ, Zhang DW. Influence of size of hematite powder on its reduction kinetics by H2 at low temperature. J Iron Steel Res Int. 2009;16:7–11.
Yu D, Zhu M, Utigard TA, Barati M. TG/DTA study on the carbon monoxide and graphite thermal reduction of a high-grade iron nickel oxide residue with the presence of siliceous gangue. Thermoch Acta. 2014;575:1–11.
Roduit B, Maciejewski M, Baiker A. Influence of experimental conditions on the kinetic parameters of gas-solid reactions -parametric sensitivity of thermal analysis. Thermochim Acta. 1996;82–283:101–19.
Piotrowski K, Wiltowskim T, Mondal K, Stonawski L, Szymanski T, Dasgupta D. Simultaneous influence of gas mixture composition and process temperature on Fe2O3–FeO reduction kinetics—neural. Braz J Chem Eng Netw Model. 2005;22:419–32.
Jung SS, Lee JS. In-situ Kinetic study of hydrogen reduction of Fe2O3 for the production of fe nanopowder. Mater Trans. 2009;50:2270–6.
Zhao W, Chen H, Liu N, Zhou J. Thermogravimetric analysis of peat decomposition under different oxygen concentrations. J Therm Anal Calorim. 2014;117:489–97.
Muraleedharan K, Mallikassery JJ, Sarada K, Kannan MP. Isothermal decomposition of K2C2O4. J Therm Anal Calorim. 2014;116:1055–60.
Jankovic BZ, Jankovic MM. Thermal characterization and isothermal kinetic analysis of commercial Creosote decomposition process. J Therm Anal Calorim. 2014;115:823–32.
Maqueda LAP, Ortega A, Criado JM. The use of master plots for discriminating the kinetic model of solid state reactions from a single constantrate thermal analysis (CRTA) experiment. Thermochim Acta. 1996;277:165–73.
Chen F, Sorensen OT, Meng G, Peng D. Thermal decomposition of BaC2O4.0, 5H2O studied by stepwise isothermal analysis and non-isothermal thermogravimetry. J Therm Anal. 1998;53:397–410.
Sherchenkov A, Kozyukhin S, Babich A. Estimation of kinetic parameters for the phase change memory materials by DSC measurements. J Therm Anal Calorim. 2014;. doi:10.1007/s10973-014-3899-8.
Bessières J, Bessières A, Heizmann JJ. Iron oxide reduction kinetics by hydrogen. Int J Hydrog Energy. 1980;5:585–95.
Lina HY, Chena YW, Li C. The mechanism of reduction of iron oxide by hydrogen. Thermochim Acta. 2003;400:61–7.
Vieira CMF, Andrade PM, Maciel GS, Vernilli F Jr, Monteiro SN. Incorporation of fine steel sludge waste into red ceramic. Mater Sci Eng, A. 2006;427:142–7.
Yi C, Chen BY, Wang CR, Ke JX. Experimental research on reducing the dust of BOF in CO2 and O2 mixed blowing steelmaking process. ISIJ Int. 2009;49:1694–9.
Andrade PM, Vieira CMF, Monteiro SN, Vernilli F Jr. Recycling of steel sludge into red ceramic. Mater Sci Forum. 2006;530:544–9.
Hughes R, Kam EKT, Mogadam-Zadeh H. The reduction of iron ores by hydrogen and carbon monoxide and their mixtures. Thermoch Acta. 1982;59:361–77.
Wang H, Yang Y, Wu BS, Xu J, Ding MY, Wang HL, Fan WH, Xiang HW, Li YW. Hydrogen reduction kinetics modeling of a precipitated iron Fischer–Tropsch catalyst. J Mol Catal A: Chem. 2009;308:96–107.
Pineau A, Kanari N, Gaballah I. Kinetics of reduction of iron oxides by H2 Part I: low temperature reduction of hematite. Thermochim Acta. 2006;447:89–100.
Pineau A, Kanari N, Gaballah I. Kinetics of reduction of iron oxides by H2 Part II. low temperature reduction of magnetite. Thermochim Acta. 2007;456:75–88.
Acknowledgements
The authors would like to thank FAPESP (The State of São Paulo’s Research Support Foundation) process 11/51638-0, the CNPq (National Council for Scientific and Technological Development) process 245470/2012-3, the CAPES (Coordination for the Improvement of Higher Education Personnel) Foundation, project PE003/2008 and the University of São Paulo.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Junca, E., Restivo, T.A.G., Espinosa, D.C.R. et al. Application of stepwise isothermal analysis method in the kinetic study of reduction of basic oxygen furnace dust. J Therm Anal Calorim 120, 1913–1919 (2015). https://doi.org/10.1007/s10973-015-4491-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4491-6