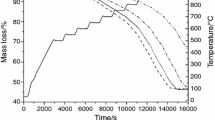
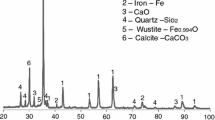
Abstract
The purpose of this paper is to study the kinetic reaction of electric arc furnace dust pellets using a reducing gas simulating the reformed natural gas. The kinetic investigation was accomplished via forced stepwise isothermal analysis method. The tests were programmed in the range of 500–1000 °C. It was used a reducing gas contained 25 % of CO and 75 % of H2. X-ray patterns were analyzed in order to understand the reduction process. The results show the reduction of electric arc furnace dust pellet occurs in three steps. At 550–650 °C, the zinc ferrite is reduced to magnetite and zinc oxide, followed by the reduction of magnetite to wustite. Carbon deposition on the pellet surface was detected in this step, which impairs the kinetic analysis. In the range of 700–800 °C, wustite is reduced to iron. It was determined a mixed control between nucleation and diffusion with E a of 89.2 kJ mol−1. At 850–950 °C, the main reaction is zinc oxide reduction to gaseous zinc. However, wustite reduction is also observed. The E a of 135.5 kJ mol−1 with a mixed control between diffusion and chemical reaction was detected.








Similar content being viewed by others
References
Oustadakis P, Tsakiridis PE, Katsiapi A, Leonardou SA. Hydrometallurgical process for zinc recovery from electric arc furnace dust (EAFD) Part I: characterization and leaching by diluted sulphuric acid. J Hazard Mater. 2010;179:1–7.
Best TE, Pickles CA. In-flight plasma reduction of electric arc furnace dust in carbon monoxide. Can Metall Q. 2001;40:61–78.
Maslehuddin M, Awan FR, Shameema M, Ibrahimam M, Ali MR. Effect of electric arc furnace dust on the properties of OPC and blended cement concretes. Constr Build Mater. 2011;25:308–12.
Jarupisitthorn C, Pimtong T, Lothongkum G. Investigation of kinetics of zinc leaching from electric arc furnace dust by sodium hydroxide. Mater Chem Phys. 2002;77:531–5.
Carranza F, Romero R, Mazuelos A, Iglesias N. Recovery of Zn from acid mine water and electric arc furnace dust in an integrated process. J Environ Manag. 2016;165:175–83.
Salihoglu G, Pinarli V. Steel foundry electric arc furnace dust management: stabilization by using lime and Portland cement. J Hazard Mater. 2008;153:1110–6.
Menad N. Ayala JN, Carcedo FG, Hernández A. Study of the presence of fluorine in the recycled fractions during carbothermal treatment of EAF dust. Waste Manag. 2003;23:483–91.
Grillo FF, Coleti JL, Espinosa DCR, Oliveira JR, Tenório JAS. Zn and Fe recovery from electric arc furnace dusts. Mater Trans. 2014;55:351–6.
Martins FM, Neto JMR, Cunha CJ. Mineral phases of weathered and recent electric arc furnace dust. J Hazard Mater. 2008;154:417–25.
Soilic T, Mioc AR, Stefanovic SC, Radovic VN, Jenko M. Characterization of steel mill electric-arc furnace dust. J Hazard Mater B. 2001;109:59–70.
Machado JG, Brehm FA, Moraes CA, Santos CA, Vilela AC, Cunha JB. Chemical, physical, structural and morphological characterization of the electric arc furnace dust. J Hazard Mater B. 2006;136:953–60.
Kukurugya F, Vindt T, Havlík T. Behavior of zinc, iron and calcium from electric arc furnace (EAF) dust in hydrometallurgical processing in sulfuric acid solutions: thermodynamic and kinetic aspects. Hydrometallurgical. 2015;154:20–32.
Lee JJ, Lin CI, Chen HK. Carbothermal reduction of zinc ferrite. Mater Trans B. 2001;32B:1033–40.
Tong LF. Reduction mechanisms and behaviour of zinc ferrite-Part 1: pure ZnFe2O4. Min Process Extr Metall. 2001;110:14–24.
Wu CC, Chang FC, Chen W, Tsai MS, Wang YN. Reduction behavior of zinc ferrite in EAF-dust recycling with CO gas as a reducing agent. J Environ Manag. 2014;143:208–13.
Junca E, Oliveira JR, Restivo TAG, Espinosa DCR, Tenório JAS. Synthetic zinc ferrite reduction by means of mixtures containing hydrogen and carbon monoxide. J Therm Anal Calorim. 2015;123:631–41.
Tzanetis KF, Martavaltzi CS, Lemonidou AA. Comparative energy analysis of sorption enhanced and conventional methane steam reforming. Int J Hydrog Energy. 2012;37:16308–20.
Angeli SD, Monteleone G, Giaconia A, Lemonidou AA. State-of-the-art catalysts for CH4 steam reforming at low temperature. Int J Hydrog Energy. 2014;39:1979–97.
Esen V, Oral B. Natural gas reserve/production ratio in Russia, Iran, Qatar and Turkmenistan: a political and economic perspective. Energy Policy. 2016;93:101–9.
Junca E, Oliveira JR, Restivo TAG, Espinosa DCR, Tenório JAS. Application of stepwise isothermal analysis method in the kinetic study of reduction of basic oxygen furnace dust. J Thermal Anal Calorim. 2015;120:1913–9.
Husum PL, Sorensen OT. Computer controlled forced stepwise isothermal analysis. Termochim Acta. 1987;114:131–8.
Junca E, Espinosa DCR, Tenório JAS. Characterization of dust generated in electric arc furnace. Int Conf Solid Waste Technol Manag. 2012;27:700–7.
Pineau A, Kanari N, Gaballah I. Kinetics of reduction of iron oxides by H2: part I: low temperature reduction of hematite. Thermochim Acta. 2006;447:89–100.
Jozwiak WK, Kaczmarek E, Maniecki TP, Ignaczak W, Maniukiewicz W. Reduction behavior of iron oxides in hydrogen and carbon monoxide atmospheres. Apll Catal A Gen. 2007;326:17–27.
Kim WH, Lee S, Kim SM, Min DJ. The retardation kinetics of magnetite reduction using H2 and H2 e H2O mixtures. Int J hydrog Energy. 2013;38:4194–200.
Roduit B, Maciejewski M, Baiker A. Influence of experimental conditions on the kinetic parameters of gas–solid reactions -parametric sensitivity of thermal analysis. Thermochim Acta. 1996;282–283:101–19.
Janković B, Smičiklas I, Stajić-Trošić J, Antonović D. Thermal characterization and kinetic analysis of non-isothermal decomposition process of bauxite red mud. estimation of density distribution function of the apparent activation energy. Int J Miner Process. 2013;123:46–59.
Chen F, Sorensen OT, Meng G, Peng D. Thermal decomposition of BaC2O4 0.5H2O studied by stepwise isothermal analysis and non-isothermal thermogravimetry. J Therm Anal. 1998;53:397–410.
Wimmers OJ, Arnoldy P, Moulijn JA. Determination of the reduction mechanism by temperature-programmed reduction: application to small FeO particles. J Phys Chem. 1986;90:1331–7.
Lin HY, Chen YW, Li C. The mechanism of reduction of iron oxide by hydrogen. Thermochim Acta. 2003;400:61–7.
Tanaka H. Thermal analysis and kinetics of solid state reactions. Thermochim Acta. 1995;267:29–44.
Maqueda LAP, Ortega A, Criado JM. The use of master plots for discriminating the kinetic model of solid state reactions from a single constantrate thermal analysis (CRTA) experiment. Thermochim Acta. 1996;277:165–73.
Vyazovkin S, Chrissafis K, Lorenzo ML, Koga N, Pijolat M, Roduit B, Sbirrazzuoli N, Suñol JJ. ICTAC kinetics committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochim Acta. 2014;59:1–23.
Silva MC, Bernardes AM, Bergmann CP, Tenório JAS, Espinosa DCR. Characterization of electric arc furnace dust generated during plain carbon steel production. Ironmak Steelmak. 2008;35:315–20.
Nyrenda RL. The processing of steelmaking flue-dust: a review. Miner Eng. 1991;4:1003–25.
Yu D, Zhu M, Utigard TA, Barati M. TG/DTA study on the carbon monoxide and graphite thermal reduction of a high-grade iron nickel oxide residue with the presence of siliceous gangue. Themochim Acta. 2014;575:1–11.
Yu D, Zhu M, Utigard TA, Barati M. TGA kinetic study on the hydrogen reduction of an iron nickel oxide. Miner Eng. 2013;54:32–8.
Hughes R, Kam EKT, Zadeh HM. The reduction of iron ores by hydrogen and carbon monoxide and their mixtures. Thermochim Acta. 1982;59:361–77.
Zhang T, Lei C, Zhu Q. Reduction of fine iron ore via a two-step fluidized bed direct reduction process. Powder Technol. 2014;254:1–11.
Pineau A, Kanari N, Gaballah I. Kinetics of reduction of iron oxides by H2: part II. Low temperature reduction of magnetite. Thermochim Acta. 2007;456:75–88.
Guger CE, Manning FS. Kinetics of zinc oxide reduction with carbon monoxide. Metall Trans. 1971;2:3083–90.
González G, Jordens ZS, Escobedo S. Dependence of zinc oxide reduction rate on the CO concentration in CO/CO2 mixtures. Thermochim Acta. 1996;278:129–34.
Weiss B, Sturn J, Voglsam S, Strobl S, Mali H, Winter F, Schenk J. Structural and morphological changes during reduction of hematite to magnetite and wustite in hydrogen rich reduction gases under fluidised bed conditions. Ironmak Steelmak. 2011;38:65–73.
Acknowledgements
The authors would like to thank FAPESP (the State of São Paulo’s Research Support Foundation) process 11/51638-0, the CNPq (National Council for Scientific and Technological Development) process 245470/2012-3, the CAPES (Coordination for the Improvement of Higher Education Personnel) Foundation, project PE003/2008, FAPES (the State of Espírito Santo’s Research and innovation Support Foundation), process 68853777/14, University of São Paulo and Federal Institute of Education, Science and Technology of Espírito Santo.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Junca, E., Restivo, T.A.G., de Oliveira, J.R. et al. Reduction of electric arc furnace dust pellets by simulated reformed natural gas. J Therm Anal Calorim 126, 1889–1897 (2016). https://doi.org/10.1007/s10973-016-5629-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5629-x