Abstract
Terazosin (C19H25N5O4, MW = 387) drug is a selective alpha 1 antagonist. It is used for lowering the blood pressure. Also, it used for treatment of symptoms of an enlarged prostate and is therefore a drug of choice for men with hypertension and prostate enlargement. In the present study, mass spectrometry (MS) and thermal analyses (TA) were used to investigate the fragmentation decomposition pathways of terazosin and confirmed by semi-empirical molecular orbital (MO) calculation, using PM3 procedure on the neutral and the positively charged species of the drug. These calculations included, bond length, bond order, partial charge distribution, ionization energy and heats of formation (ΔH f). The mass spectra and TA fragmentation pathways were proposed and compared to each other to select the most suitable scheme representing the correct fragmentation pathway of the drug in both techniques. This selection helps understanding of metabolism of the drug in vivo system. Therefore, comparison between MS and TA helps in selection, the proper pathway representing the fragmentation of this drug. This comparison successfully confirmed by MO calculation.
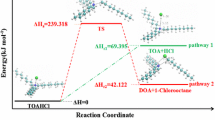
Graphical Abstract
In the present study, mass spectrometry (MS) and thermal analyses (TA) were used to investigate the fragmentation decomposition pathways of terazosin and confirmed by semi-empirical molecular orbital (MO) calculations. The mass spectra and thermal analyses fragmentation pathways were proposed and compared to each other to select the most suitable scheme representing the correct fragmentation pathway of the drug in both techniques. This selection helps understanding of metabolism of the drug in vivo system. Terazosin hydrochloride (C19H25N5O4) drug (marketed ITRIN), has an IUPAC name; 2-[4-(2-tetrahydrofuranyl) carbonyl]-1 piperazinyl- 6,7-dimethoxy-4-quinazolinamine monohydrochloride dihydrate, and its stereo structure is given below







Similar content being viewed by others
References
Ganjali MR, Faridbod F, Larijani B, Riahi S, Hosseini M, Esfahani EN, Norouzi P. Terazosin potentiometric sensor for quantitative analysis of terazosin hydrochloride in pharmaceutical formulation based on computational study. Int J Electrochem Sci. 2010;5:200–14.
Shrivastava A, Gupta VB. Stability-indicating RP-HPLC method for the simultaneous determination of prazosin, terazosin, and doxazosin in pharmaceutical formulations. Sci Pharm. 2012;80(3):619–31.
Larsen BS, Ewen CNM. Mass spectrometry of biological materials. New York: Marcel Dekker; 1998.
Levsen K. Fundamental aspects of organic mass spectrometry. Weiheim: Verlag Chemie; 1978.
Zayed MA, Fahmey MA, Hawash MF. Investigation of diazepam drug using thermal analyses, mass spectra and semi-empirical MO calculations. Spectrochim Acta A. 2005;61:799–805.
Das KG, James EP. Organic mass spectrometry. New Delhi: Oxford and IB 11 Publishing Co; 1976.
Barbas R, Prohens R, Puigjaner C. A new polymorph of norfloxacin complete characterization and relative stability of its trimorphic system. J Therm Anal Calorim. 2007;89(3):687–92.
Zayed MA, Hawash MF, Fahmey MA. Structure investigation of codeine drug using mass spectra, thermal analyses and semiempirical MO calculations. Spectrochim Acta A. 2005;64:363–71.
Sovizi MR. Investigation on decomposition kinetic of naproxen and celecoxib. J Therm Anal Calorim. 2010;102:285–9.
Santos AFO, Basílio ID Jr, de Souza FS, Medeiros AFD, Pinto MF, de Santana DP, Macêdo RO. J Application of thermal analysis in study of binary mixtures with metformin. Therm Anal Calorim. 2008;93(2):361–4.
Michalik K, Drzazga Z, Michnik A. Calorimetric characterization of 2′,3′-dideoxyinosine water solution. J Therm Anal Calorim. 2008;93(2):521–6.
Pourmortazavi SM, Hajimirsadeghi SS, Hosseini SG. Characterization of the aluminium/potassium chlorate mixtures by simultaneous TG-DTA. J Therm Anal Calorim. 2006;84:557–61.
Lever SD, Papadaki M. Study of condition-dependent decomposition reactions; part I. The thermal behavior and decomposition of 2-nitrobenzoyl chloride. J Hazard Mater. 2004;115(1–3):91–100.
Pourmortazavi SM, Hosseini SG, Hajimirsadeghi SS, Fareghi Alamdari R. Investigation on thermal analysis of binary zirconium/oxidant pyrotechnic systems. Combust Sci Tech. 2008;180:2093–102.
Hosseini SG, Pourmortazavi SM, Hajimirsadeghi SS. Thermal decomposition of pyrotechnic mixtures containing sucrose with either potassium chlorate or potassium perchlorate. Combust Flame. 2005;141:322–6.
Fahmy MA, Zayed MA, Keshk YH. Comparative study on the fragmentation of some simple phenolic compounds using mass spectrometry and thermal analyses. Thermochim Acta. 2001;366:183–8.
Fahmey MA, Zayed MA. Phenolic-iodine redox products. Mass spectrometry, thermal analysis and other physicochemical methods of analyses. J Therm Anal Calorim. 2002;67:163–75.
Fahmey MA, Zayed MA, El-Shobaky HG. Study of some phenolic-iodine redox polymeric products by thermal analyses and mass spectrometry. J Therm Anal Calor. 2005;82:137–42.
Somogyi Á, Harrison AG, Paizs B. Using gas-phase guest-host chemistry to probe the structures of b ions of peptides. J Am Soc Mass Spectrom. 2012;23:2055–8.
Zayed MA, Hawash MF, Fahmey MA, El-Gizouli AMA. Investigation of ibuprofen drug using mass spectrometry, thermal analyses and semi-empirical molecular orbital calculation. J Therm Anal Calorim. 2012;108:315–22.
Zayed MA, Mohamed GG, Fahmey MA. Thermal and mass spectral characterization of novel azo dyes of p-acetoamidophenol in comparison with Hammett substituent effects and molecular orbital calculations. J Therm Anal Calorim. 2012;107:763–76.
Frag EYZ, Zayed MA, Omar MM, Elashery SEA, Mohamed GG. Spectrophotometric determination of carbamazepine and mosapride citrate in pure and pharmaceutical preparations. Arabian J Chem. 2012;5:375–82.
Stewart JJP. Optimization of parameters for semiempirical methods I. J Comput Chem. 2004;10(2):209–20.
Baker J. An algorithm for the location of transition states. J Comput Chem. 2004;7(4):385–95.
Stewart JJP. Software package MOPAC 2000. Tokyo: Fujitsu Limited; 1999.
Stanley SM. Equine metabolism of buspirone studied by high-performance liquid chromatography/mass spectrometry. J Mass Spectrom. 2000;35:402–7.
Zayed EM, Zayed MA, Hindy AMM. Thermal and spectroscopic investigation of novel Schiff base, its metal complexes, and their biological activities. J Therm Anal Calorim. 2014;116:391–400.
Loew G, Chadwick M, Smith D. Applications of molecular orbital theory in organic molecules. Org Mass Spectrom. 1973;7:1241.
Cooks RG, Beynon JH, Caprioli RM, Laster GR. Metastable Ions. Amsterdam: Elsevier; 1973.
Ey O, Base SK, Kwon JW, You M, Lee DC III, Lee MG. Pharmacokinetic and pharmacodynamics consequences of inhibition of terazosin metabolism via CYP3A1 and/or 3A2 by DA-8159, an erectogenic, in rats. Br J Pharmacol. 2007;151:24–34.
Cheah PY, Yuen KH, Liong ML. Improved high-performance liquid chromatographic analysis of terazosin in human plasma. J Chromatogr B Biomed Sci Appl. 2000;745(2):439–43.
Vincent J, Dachman W, Blaschke TF, Hoffman BB. Pharmacological tolerance to alpha 1-adrenergic receptor antagonism mediated by terazosin in humans. J Clin Investig. 1992;90(5):1763–8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zayed, M.A., Fahmey, M.A., El-Desawy, M. et al. Structure characterization of terazosin drug using mass spectrometry and thermal analyses techniques in comparison with semi-empirical molecular orbital (MO) calculations. J Therm Anal Calorim 120, 1061–1069 (2015). https://doi.org/10.1007/s10973-015-4462-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4462-y