Abstract
Abstract
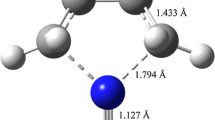
The thermal dissociation of tri-n-octylamine hydrochloride (TOAHCl) was investigated using both the quantum chemical simulation and experimental methods. The pathway through which a mixture of tri-n-octylamine (TOA) and hydrogen chloride (HCl), rather than di-n-octylamine (DOA) and 1-chlorooctane, are produced has been determined through transition state (TS) search with Intrinsic Reaction Coordinate (IRC) calculations. Particularly, strong agreement between the experimental FTIR spectra and that of TOA demonstrates the same result for the first time. Moreover, the thermal dissociation of TOAHCl proceeds in two continuous steps, which is different from the low molecular mass amine hydrochlorides. The experimental enthalpy of the dissociation was 70.793 \(\hbox {kJ mol}^{-1}\) with DSC measurement which is very close to the density functional theory (DFT) calculation result 69.395 \(\hbox {kJ mol}^{-1}\). Furthermore, with the aid of DFT calculations, some other important thermochemical characteristics such as crystal lattice energy with the value of 510.597 \(\hbox {kJ mol}^{-1}\) were evaluated by means of Born–Fajans–Haber cycle.
Graphical Abstract
The thermal dissociation of tri-n-octylamine hydrochloride was investigated with both the quantum chemical simulation and experimental methods. Intrinsic reaction coordinate (IRC) calculations suggest that the products are tri-n-octylamine and HCl rather than di-n-octylamine and 1-chlorooctane. Furthermore, the dissociation is a two-stage process as determined by experimental analysis.









Similar content being viewed by others
References
Sarangi K, Padhan E, Sarma P V R B, Park K H and Das R P 2006 Removal/recovery of hydrochloric acid using Alamine 336, Aliquat 336, TBP and Cyanex 923 Hydrometallurgy 84 125
Kojima T, Fukutomi H and Kakihana H 1969 Extraction of hydrochloric acid by tri-n-octylamine in benzene B. Chem. Soc. Jpn. 42 875
Schunk A and Maurer G 2003 Distribution of hydrochloric, nitric, and sulfuric acids between water and organic solutions of tri-n-octylamine Part I. Toluene as organic solvent Fluid Phase Equilibria 207 1
Schunk A and Maurer G 2003 Distribution of hydrochloric, nitric, and sulfuric acids between water and organic solutions of tri-n-octylamine Part II. Methylisobutylketone as organic solvent Fluid Phase Equilibria 21 189
Agrawal A and Sahu K K 2009 An overview of the recovery of acid from spent acidic solutions from steel and electroplating industries J. Hazard. Mater. 171 61
Nguyen T H and Lee M S 2014 Extraction and stripping of inorganic acids by tris-2-ethylhexyl amine J. Korean Inst. Met. Mater. 52 799
Banda R, Nguyen T H, Sohn S H and Lee M S 2013 Recovery of valuable metals and regeneration of acid from the leaching solution of spent HDS catalysts by solvent extraction Hydrometallurgy 133 161
Banda R, Nguyen T H and Lee M S 2013 Recovery of HCl from chloride leach solution of spent HDS catalyst by solvent extraction Chem. Process Eng. 34 153
Fu X, Liu H, Chen H, Hu Z and Wang D 1999 Three-phase extraction study of the TOA-alkane/HCl (\(\text{ Zn }^{2+}\) or \(\text{ Fe }^{3+})\) systems Solv. Extr. Ion. Exch. 17 1281
Li Y, Song X, Chen G, Sun S, Xu Y and Yu J 2015 Extraction of hydrogen chloride by a coupled reaction-solvent extraction process Front. Chem. Sci. Eng. 9 479
Li Y, Song X, Chen G, Sun Z, Xu Y and Yu J 2015 Preparation of calcium carbonate and hydrogen chloride from distiller waste based on reactive extraction–crystallization process Chem. Eng. J. 278 55
Liu X, Wang W, Wang M and Wang P 2014 Experimental Study of \(\text{ CO }_{2}\) mineralization in \(\text{ Ca }^{2+}\)-rich aqueous using tributylamine as an enhancing medium Energ. Fuel. 28 2047
Wang W, Liu X, Wang P, Zheng Y and Wang M 2013 Enhancement of \(\text{ CO }_{2}\) mineralization in \(\text{ Ca }^{2+}\)-\(/\text{ Mg }^{2+}\)-rich aqueous solutions using insoluble amine Ind. Eng. Chem. Res. 52 8028
Chen G, Song X, Dong C, Sun S, Sun Z and Yu J 2016 Mineralizing \(\text{ CO }_{2}\) as \(\text{ MgCO }_{3}\cdot \text{3H }_{2}\text{ O }\) Using Abandoned \(\text{ MgCl }_{2}\) Based on a Coupled Reaction–Extraction–Alcohol Precipitation Process Energ. Fuel. 30 7551
Xu X and Zhu T 2005 Coupled process of reaction and solvent extraction I. The reaction between \(\text{ CO }_{2}\) and \(\text{ SrCl }_{2}\) coupled with solvent extraction of HCl Hydrometallurgy 76 11
Zhou Z, Liang F, Qin W and Fei W 2014 Coupled reaction and solvent extraction process to form \(\text{ Li }_{2}\text{ CO }_{3}\): mechanism and product characterization AIChE J. 60 282
Agrawal A 2007 Extraction of acid and iron values from sulphate waste pickle liquor of a steel industry by solvent extraction route Hydrometallurgy 88 55
Huang Q, Jin F, and Kang S 2007 Production of potash by amine extraction Chem. Ind. Eng. 24 304 (in Chinese)
Kang H, Zhou X, Dong L and Feng T 2011 Synergetic extraction of phytic acid from HCl extract of rapeseed meal with Alamine 336 and n-octanol dissolved in sulfonated kerosene Ind. Eng. Chem. Res. 50 8658
Eyal A and Banlel A 1982 Extraction of strong mineral acids by organic acid-base couples Ind. Eng. Chem. Proc. Dd. 21 334
Zhang J, Zhang R, Geerlings H and Bi J 2010 A novel indirect wollastonite carbonation route for \(\text{ CO }_{2}\) sequestration Chem. Eng. Technol. 33 1177
Coenen A, Kosswig K and Prominski G 1978 Process for obtaining gaseous hydrogen chloride from dilute, aqueous hydrochloric acid U.S. Patent 4115530
Coenen A, Kosswig K, Hentschel B and Ziebarth J 1980 Method of manufacturing hydrogen chloride from solutions of amine hydrochlorides U.S. Patent 4230681
Eyal A M and Baniel A M 1991 Recovery and concentration of strong mineral acids from dilute solutions through LLX. I. Review of parameters for adjusting extractant properties and analysis of process options Solvent Extr. Ion Exc. 9 195
Hentschel B, Ziebarth J, Coenen A, Kosswig K and Praun F V 1982 Method for preparing sodium bicarbonate and hydrogen chloride U.S. Patent 4337234
Baniel A and Eyal A 2010 Process for the recovery of HCl from a dilute solution thereof U.S. Patent 2010/0093995 A1
Baniel A and Eyal A 2011 Process for the recovery of HCl from a dilute solution thereof and extractant composition for use therein U.S. Patent 2011/0028710 A1
Błazejowski J, Szychlinski J and Kowalewska E 1993 Thermal reactions of lead(iv) chloride complexes in the solid state. Part V. Thermolysis of mono-n-alkylammonium hexachloroplumbates Thermochim. Acta 66 197
Blażejowski J 1983 Thermal properties of amine hydrochlorides. Part I. Thermolysis of primary n-alkylammonium chlorides Thermochim. Acta 68 233
Rodebush W H and Michalek J C 1929 The vapor pressure and vapor density of intensively dried ammonium chloride J. Am. Chem. Soc. 51 748
Harrison R M, Sturges W T, Kitto A M N and Li Y 1990 Kinetics of evaporation of ammonium chloride and ammonium nitrate aerosols Atmos. Environ. 24A1883
Blażejowski J and Kowalewska E 1986 Thermal properties of amine hydrochlorides. Part II. Thermolysis and thermochemistry of alkanaminium chlorides Thermochim. Acta 105 257
Błazejowski J, Krzymiñski K, Storoniak P and Rak J 2000 Thermodynamics and kinetics of the thermal decomposition of N,N,N-Trimethylmethanaminium and 1-Methylpyridinium halides J. Therm. Anal. Calorim. 60 927
Sawicka M, Błazejowski J and Rak J 2006 TG-FTIR, DSC and quantum chemical studies of the thermal decomposition of quaternary methylammonium halides Chem. Phys. 324 425
Sawicka M, Błazejowski J and Rak J 2006 TG-FTIR, DSC, and quantum-chemical studies on the thermal decomposition of quaternary ethylammonium halides J. Phys. Chem. A 110 5066
Mills G and Jónsson H 1994 Quantum and thermal effects in \(\text{ H }_{2}\) dissociative adsorption: Evaluation of free energy barriers in multidimensional quantum systems Phys. Rev. Lett. 72 1124
Henkelman G and Jónsson H 2000 Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points J. Chem. Phys. 113 9978
Hutter J Iannuzzi M Schiffmann F and Vondele J V 2014 WIREs Comput. Mol. Sci. 4 15
Frisch M J, Trucks G W, Schlegel H B, et al. Gaussian 09, revision D.01; Gaussian, Inc.: Wallingford CT, 2009.
Larkin P J 2011 In Infrared and Raman Spectroscopy: Principles and Spectral Interpretation P J Larkin (Ed.) (Elsevier Inc.: Waltham MA) p. 75, 76, 78, 107, 109, 120, 126, 157, 158
Weng S In Fourier transform infrared spectrum 2nd edn. J X Du and D Xiang (Ed.) (Chemical Industry Press: Beijing) p. 338 (in Chinese)
Gibson E K 2007 Amine hydrochloride salts: a problem in polyurethane synthesis (University of Glasgow, Glasgow, UK) pp. 98–100, 103
Bauer S H, Yamazaki T, Lazaar K I and Chiu N S 1985 Intermolecular conversion over a low barrier. 3. Gas-phase NMR studies of an H bond association J. Am. Chem. Soc. 107 743
Latajka Z, Sakai S and Morokuma K 1984 Possible gas-phase ion pairs in amine-HCl complexes. An ab initio theoretical study Chem. Phys. Lett. 110 464
Clementi E and Gayles J N 1967 Study of the Electronic Structure of Molecules. VII. Inner and Outer Complex in the \(\text{ NH }_{4}\text{ Cl }\) Formation from \(\text{ NH }_{3}\) and HCl J. Chem. Phys. 47 3837
Brciz A, Karpfen A, Lischka H and Schuster P 1984 A candidate for an ion pair in the vapor phase: Proton transfer in complexes \(\text{ R }_{3}\text{ N }\)-HX Chem. Phys. 89337
Goldfinger P and Verhaegen G 1969 Stability of the gaseous ammonium chloride molecule J. Chem. Phys. 50 1467
de Kruif G G 1982 The vapor phase dissociation of ammonium salts: Ammonium halides, ammonium rhodanide, ammonium nitrate, and ammonium bicarbonate J. Chem. Phys. 77 6247
Barnes A J, Beech T R and Mielke Z 1984 Strongly hydrogen-bonded molecular complexes studied by matrix-isolation vibrational spectroscopy Part 1. The ammonia-hydrogen chloride complex J. Chem. Soc., Faraday Trans. 2 80 455
Barnes A J, Nicholas J, Kuzniarski S and Mielke Z 1984 Strongly hydrogen-bonded molecular complexes studied by matrix-isolation vibrational spectroscopy Part 2. Amine-hydrogen chloride complex J. Chem. Soc., Faraday Trans. 2 80 465
Steele W V, Chirico R D, Knipmeyer S E, Nguyen A, Smith N K and Tasker I R 1996 Thermodynamic properties and ideal-gas enthalpies of formation for cyclohexene, phthalan (2,5-Dihydrobenzo-3,4-furan), isoxazole, octylamine, dioctylamine, trioctylamine, phenyl isocyanate, and 1,4,5,6-tetrahydropyrimidine J. Chem. Eng. Data 41 1269
Waddington T C 1959 Lattice energies and their significance in inorganic chemistry Adv. Inorg. Chem. Radiochem. 1 157
Goodliffe A L, Jenkins H D B and Martin S V 1971 The proton affinity of gaseous ammonia, the charge distribution on the \(\text{ NH }_{4}^{+}\) ion and the lattice energies of \(\text{ NH }_{4}\text{ C1 }, \text{ NH }_{4}\text{ Br }\) and \(\text{ NH }_{4}\text{ I }\) Mol. Phys. 21 761
Lide D R 2004 In CRC Handbook of Chemistry and Physics 85\(^{{\rm th}}\) edn. D R Lide (Ed.) (London: CRC press) pp. 5-2
Valadbeigi Y, Farrokhpour H and Tabrizchi M 2014 G4MP2, DFT and CBS-Q calculation of proton and electron affinities, gas phase basicities and ionization energies of hydroxylamines and alkanolamines J. Chem. Sci. 126 1209
Acknowledgements
The authors are greatly thankful to the National High Technology Research and Development Program of China (No. 2011AA06A107) for financial support. Also, authors thank China Scholarship Council (CSC) for backing the project. Authors thank the University of Amsterdam and SURFsara in The Netherlands for offering the resources for calculation on CP2K and Gaussian 09, respectively.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
DONG, C., SONG, X., MEIJER, E.J. et al. Mechanism studies on thermal dissociation of tri-n-octylamine hydrochloride with FTIR, TG, DSC and quantum chemical methods. J Chem Sci 129, 1431–1440 (2017). https://doi.org/10.1007/s12039-017-1357-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-017-1357-4