Abstract
This paper presents the results of investigating the thermal stability, flammability, and fire hazard of cross-linked EVM/NBR blends unfilled and filled with halogenless flame-retardant compounds such as melamine cyanurate or magnesium hydroxide. The thermal analysis of the blends was carried out in the atmosphere of air. The activation energy of the composite destruction was determined by two non-isothermal methods: Flynn–Wall–Ozawa’s and Kissinger’s methods. The flammability of the composites obtained was determined by the method of oxygen index and on the basis of their combustion in air. The fire hazard of the vulcanizates investigated was determined with the use of a cone calorimeter and on the basis of toxicometric parameters W LC50SM. The test results have shown that the flame retardants used increase the thermal stability of the cross-linked blends and decrease their flammability, and thereby allow one to obtain self-extinguishing or non-flammable polymeric materials. The cross-linked EVM/NBR blends filled with these flame-retardant compounds are characterized by good mechanical properties and reduced fire hazard.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Elastomers and materials made of them are commonly used almost in all fields of live. Despite numerous ecological restrictions and considerable amounts of process and postconsumer wastes, often difficult to recycle, the production and consumption of elastomers and related materials have been systematically increased worldwide. Hence, the problem of the flammability reduction of rubber products or making them non-flammable is of special importance on account of a serious health and life hazard and environmental pollution due to considerable emission of smoke and products, especially toxic compounds, during their thermal decomposition and combustion.
From the review of literature data, it follows that 55–80 % of all the fatal accidents during fires are caused by poisoning with the products of thermal decomposition and combustion as well as smoke [1, 2]. These easily penetrate human organism as a result of inhalation and absorption by skin.
The thermal decomposition and combustion of elastomeric materials most often results in the formation of toxic compounds such as carbon oxide, hydrogen chloride, hydrogen cyanide, nitrogen and sulfur oxides, nitro-compounds, organic halogen derivatives including those of dibenzo-p-dioxins and dibenzofurans [3, 4]. A serious hazard is also the emission of polycyclic aromatic hydrocarbons (PAH) [5, 6]. The most harmful substances causing environment toxicity include the first six compounds mentioned, as they emit in the highest amounts and their effect on human organism is quick, while the values of threshold limit value-time weighed average (TLV-TWA) are very low [7].
The foundation of action aimed at the reduction in human losses is the laboratory test results including the measurements of the potential formation rate of thermal decomposition and combustion products, quantity and rate of smoke emission under set combustion conditions, heat quantity emitted, increase in oxygen deficit, and mutual relations between these parameters. Testing elastomeric materials under the same kinetic and thermal conditions, one can estimate their behavior during fire. Such tests allow one also to attempt to modify the composition of these materials in order to increase their resistance to ignition or to reduce the emission of toxic products during combustion.
This paper presents the test results concerning the thermal stability and fire hazard of the cross-linked ethylene-vinyl acetate (EVM)/NBR rubber blends. The effect of halogenless flame retardants on the thermal properties and fire hazard posed by the composites under investigation was elucidated.
Experimental
Materials
The test materials included EVM rubbers with different contents of vinyl monomeric units in the macromolecules of Levapren 450 (L450) and Levapren 800 (L800) from Lanxess Company, containing 45 ± 1.5 % and 80 ± 2 % vinyl acetate mers, respectively, and NBR 2255 V containing 22 % of combined acrylonitrile from Lanxess Company.
The mixtures of L450 or L800 with NBR (EVM/NBR: L450/NBR, L800/NBR) were prepared at ratios of 30:70 (EVM/NBR70: L450/NBR70, L800/NBR70); 50:50 (EVM/NBR50: L450/NBR50, L800/NBR50); and 70:30 (EVM/NBR30: L450/NBR30, L800/NBR30).
Mixtures EVM with NBR were cross-linked by means of dicumyl peroxide, DCP, (0.6 phr) in the presence of zinc oxide, ZnO, (5 phr).
Melamine cyanurate (CM) (POCh Gliwice), magnesium hydroxide (Martinswerk, GmbH), and Magnifin H5 (Mg), were used as flame retardant and were incorporated into L450/NBR30 or L800/NBR30 mixtures in the following quantities: melamine cyanurate 50 phr magnesium hydroxide 60 or 80 phr.
Methods
Preparing elastomer blends and their vulcanization
Mixtures EVM with NBR were prepared at room temperature with the use of a laboratory roll stand with roll dimensions D = 150 mm, L = 300 mm. The rotational speed of the front roll was 20 rpm, friction 1.1.
Blends EVM with NBR were vulcanized in steel molds placed between electrically heated press shelves. The optimal vulcanization time (τ 0.9) at a temperature of 160 °C was determined by means of a WG-2 vulcameter according to PN-ISO 3417:1994.
Thermal properties rubbers and their vulcanizates
The thermal properties of cross-linked mixtures were determined under air atmosphere within the temperature range of 25–700 °C, by means of the thermal analyzer Jupiter STA 449 F3 from Netzsch Company (weighed portions 5–10 mg, heating rate 10 °C min−1).
Thermal analysis under nitrogen at −100 to 500 °C was carried out by the method of differential scanning calorimetry using a DSC-204 micro-calorimeter (Netzsch) and 5–7 mg weighed portions at a heating rate of 10 °C min−1.
Determination of activation energy of decomposition investigation vulcanizates
The activation energy of decomposition of vulcanizates EVM/NBR and their composites containing appropriate flame retardants have been calculated by the use of Flynn–Wall–Ozawa and Kissinger’s methods.
The analysis is based on the well-known Flynn–Wall–Ozawa isoconversional method. This isoconversional integral method, suggested independently by Flynn and Wall and Ozawa uses Doyle’s approximation of the temperature integral. This method is based on the equations [8–12]:
where β is the heating rate °C min−1; A, the pre-exponential factor; E, activation energy of decomposition in kJ mole−1; R, the universal gas constant; α, the degree of conversion; T, the absolute temperature to reach the conversion; and
is the integral conversion function.
Thus, at a constant conversion (α = const.), the plot log β versus (1/T), obtained from a series of experiments performed at several heating rates, should be a straight line whose slope allows evaluation of the activation energy:
To apply this isoconversional method, heating rates of 5, 10, and 15 °C min−1 were chosen. In this study, the conversion values of 10, 20, 30, 40, 50, 60, 70, 80, and 90 % have been used, which would give α values 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, and 0.9, respectively, for the Flynn–Wall–Ozawa method.
Kissinger’s method Kissinger’s method is one of the differential methods that have been used by researches to determine the activation energy of solid state reactions from plots of the logarithm of the heating rate versus the inverse of the temperature at the maximum reaction rate in constant heating rate experiments. The advantage of this method is that even without a precise knowledge of the reaction mechanism and reaction order, the activation energy can be determined from the following equation [13, 14]:
where β is the heating rate, T max is the temperature corresponding to the inflection point of the thermal degradation curves which corresponds to the maximum rate, A is the pre-exponential factor, E is the activation energy, α max is the maximum conversion, and n is the order of the reaction. From the slope of the plot of \( \ln (\beta /T_{\hbox{max} }^{2} ) \) versus 1000/T max, E can be calculated.
Flammability
The flammability of vulcanizates was determined by the oxygen index (OI) method, using an apparatus from Fire Testing Technology Ltd and 50 × 10 × 4 mm specimens. With a constant nitrogen flow rate through a measurement column (∅ = 75 mm), amounting to 40 ± 2 mm s−1, the oxygen concentration was selected so that the way that the sample was completely burned within time t = 180 s. The sample top was ignited for 5 s by the gas burner supplied with LPG [15, 16].
We also tested flammability in air using identical samples as in the case of OI method. A sample in a vertical position was ignited with a gaseous burner for 5 s and its combustion time (t s) was measured.
The vulcanizates under investigation were examined by the use of a cone calorimeter from Fire Testing Technology Ltd. Elastomer samples with dimensions of (100 × 100 ± 1) mm and thickness of (2 ± 0.5) mm were tested at horizontal position with the heat radiant flux density 35 kW m−2. During tests, the following parameters were recorded: initial sample mass, time to ignition (TTI), sample mass during testing, total heat released (THR), effective combustion heat (EHC), average mass loss rate (MLR), heat release rate (HRR), and sample final mass.
The structure of the rubber combustion residue analyzed by means of a cone calorimeter was assessed on the basis of photographs taken under a microscope equipped with the recording software Motic Images Plus.
Toxicity
The toxicity of the gas products of vulcanizates decomposition and combustion was assessed on the basis of the quantitative chemical determination of the specific emission of CO, CO2, HCN, NO2, HCl, and SO2 at temperatures of 450, 550, and 750 °C, according to the standard PN-B-02855:1988.
The parameter that takes into account the concentration of all gases (CO, CO2, HCN, NO2, HCl, and SO2) emitted at temperatures T = 450, 550, and 750 °C is known as a toxicometric index, W LC50SM, described by Eq. 5 [17].
W LC50SM index is the arithmetic average of W LC50M(T) indices
where n is number of gaseous products, W LC50 is maximum toxic concentration of gaseous product formed during thermal decomposition and combustion of testing material in the “T” temperature
where E is emission, g g−1; \( {\text{LC}}_{50}^{30} \) is lethal concentration of gaseous product of thermal decomposition and combustion of test material which cause a 50 % lethality of test animals during 30-min exposure
Mechanical properties
The strength properties of composites were determined according to PN-ISO 37:1998, using an apparatus from ZWICK, model 1435, connected to a computer with an appropriated software and dumbbell samples with a measurement section width of 4 mm and a thickness of about mm.
Results and discussion
Thermal analysis
The L450/NBR and L800/NBR blends tested after cross-linking showed one value of glass transition temperature. In the case of L450/NBR30 and L800/NBR30 blends, it amounts to Tg = −24 and 5 °C, respectively. This indicates a good co-miscibility of the elastomers tested resulting from interelastomeric reactions.
The thermal decomposition of cross-linked blends of L450 and L800 rubbers with NBR proceeds in three stages (Fig. 1).
The first of them, at ΔT 1 = 250–400 °C, is connected with the deacetylation processes of acetic acid and thereby with the formation of unsaturated polyenes. The second stage of thermal decomposition proceeding at ΔT 2 = 400–500 °C is connected with both the thermo-oxidizing processes of unsaturated compounds resulting in the formation of aliphatic and aromatic gaseous products, and with the thermal decomposition of NBR (Fig. 2). The final stage of decomposition occurring at ΔT 3 = 500–675 °C is connected with the combustion of the cross-linked blend residue.
The content of nitrile rubber in cross-linked blends clearly influences their thermal stability. An increase in the content of a high-molecular composite component with a greater resistance to heat at elevated temperature, i.e., NBR rubber, increases the blend decomposition start temperature (parameter ΔT I and T 5 I) (Table 1) and considerably decreases the decomposition rate, dm/dt I, of the polymeric material obtained.
The parameter of thermal stability, dm/dt II, is connected with destruction processes, whose intensity also depends on the content of NBR. However, it should be emphasized that the destruction reactions are accompanied by the cyclization of adjacent nitrile groups proceeding within a wide temperature range, which influences not only the combustion temperature of the thermal decomposition residue, ΔT s, and parameter P 700 (Table 1), but also the energy of destruction activation, especially that of the first thermal decomposition stage of the cross-linked blends (Table 2).
The incorporation of conventional halogenless flame retardants, such as melamine cyanurate or Magnifin H5 into L450/NBR30 and L800/NBR30 blends decisively changes their thermal stability (Table 3). Under the influence of magnesium hydroxide, the temperature of the beginning of blend thermal decomposition increases by about 40 °C regardless of the type of Levapren (Table 3). The thermal stability factors, T 5 I and T 50 I, are also considerably increased. It should be also mentioned that the maximal temperature of thermal decomposition, T RMAX II, indicating the intensity of destruction processes, is shifted to the range of higher temperatures.
An important parameter determining both the thermal stability of a polymeric composite and its flammability is the thermal decomposition rate, dm/dt. A decrease in the decomposition rate of polymeric materials exerts a positive influence on the reduction in their flammability. A decrease in the decomposition rate of polymeric materials exerts a positive influence on their flammability. This results from the formation of lower quantities of volatile, flammable products of pyrolysis passing into flame, which reduces the rate of free-radical reactions proceeding in it. The flame retardants used clearly influence the values of parameters dm/dt I and dm/dt II. The reduction in the first stage decomposition rate of the composites tested results first of all from the decrease in the concentration of their vinyl-acetate monomeric units under the influence of the flame retardant used. Lower values of parameter dm/dt II also result from a lowered segmental mobility of polymeric chains due to mutual interactions of macromolecules with the flame-retardant fillers.
The presence of flame retardants in the composites tested also influences the value of the destruction activation energy, especially that of macromolecules containing unsaturated bonds (the second stage of thermal decomposition). Parameter Ea clearly increases in the presence of melamine cyanurate, and especially in the presence of magnesium hydroxide (Table 4, Figs. 3, 4, 5, 6, 7), which confirms that the flame-retardant compounds used fulfill the function of screens protecting the polymer against the action of elevated temperature.
Flammability and fire hazard
From the point of view of a reduction in flammability, both the combustion (parameter P700) and thermal decomposition (Pw) residues play a significant function (Table 3). The latter parameter is becoming more and more important. The most effective flame retardants are those which during the combustion of polymeric materials bring about the formation of a carbonized residue instead of pyrolysis products or with their minimal portion [18].
The comparative analysis of the test results obtained by the method of thermal analysis in air (Table 3) leads to a conclusion that melamine cyanurate does not exert any significant effect on the thermal decomposition residue of the composite investigated compared to magnesium hydroxide. However, each of the flame retardants used considerably reduces the flammability of the polymeric materials obtained. Under the influence of melamine cyanurate, the L450/NBR30 composite is extinguished after 62 s (parameter t s, Table 5), while in the presence of magnesium hydroxide after 47 s. The increased content of Mg(OH)2 in the blend tested from 50 to 60 phr results in a nonflammable material (Table 5). Regardless of the type and content of the flame retardant, the cross-linked L800/NBR30 composite does not ignite (t s, Table 5).
It should be also emphasized that the self-extinguishing polymeric materials obtained ignited in air under the influence of flame source within 30 s. The nonflammable polymeric materials obtained also neither burned nor glowed under the gas burner flame acting for 30 s.
An important parameter for comparing the flammability of polymeric materials is OI. Materials for which OI < 0.21 (oxygen content in the combustion atmosphere is below 21 %) belong to inflammable materials, while those, whose OI > 0.28 are considered to be nonflammable. From the data listed in Table 5, it follows that taking into account the values of OI, all the materials tested, containing the flame retardant, should be rated among flame retardant and nonflammable materials [19, 20].
Evaluating the fire-safety of polymeric materials only on the basis of OI, one should act with due caution. The assumption that a material showing OI > 0.28 should not burn in air, is not under laboratory conditions a sample burns with a flame similar to that of a candle, while in practice, combustion most often proceeds from below upward. During such combustion, if the direction of flame movement and that of oxygen/nitrogen mixture flux are the same, successive sample portions are intensively heated by the heat flux flowing upwards, which facilitates combustion and decreases the value of OI.
To determine the fire hazard of the materials investigated under real fire conditions, blend samples without and with added proper flame retardants were tested with the use of a cone calorimeter (Tables 6, 7).
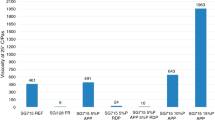
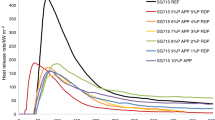
From the data listed in Table 6, it follows that among the L450/NBR and L800/NBR composites tested, L450/NBR30 and L800/NBR30 blends are characterized by the lowest fire hazard expressed by TTI, total heat emitted, heat emission rate, and maximal heat emission rate as well as ECH and MLR (Table 6; Figs. 8, 9).
The considerably reduced fire hazard of L450/NBR30 and L800/NBR30 composites in relation to the remaining blends results from the higher concentration of vinyl-acetate monomeric units in the volume unit. These units are deacetylated releasing acetic acid and diluting the flammable, often toxic products of the blend thermal decomposition.
The fire hazard of the cross-linked L450/NBR30 and L800/NBR30 blends decreases under the influence of halogenless flame retardants (Table 7).
Taking into account the quantity of flame-retardant incorporated into the blend and the fire hazard parameters, such as total emitted heat, heat emission rate, and mass loss rate, it should be concluded that melamine cyanurate shows a similar effectiveness of reducing fire hazard to that of magnesium hydroxide. The reduction in the fire hazard of the blends tested by the halogenless flame retardants (Figs. 10,11, 12, 13) may be caused by their action in both gaseous and solid phases [21–24].
The flame-retarding effect of melamine cyanurate is connected with its endothermic decomposition (cooling effect), release of nonflammable ammonia, and formation of condensation products, such as melam, melem, and melon, whose presence in the boundary layer formed during the combustion of elastomeric blend impedes the transport of mass and energy between the solid and gaseous phases of the material under combustion, dilutes volatile destruction products (Fig. 14a, c) [25, 26].
Water vapor released during the decomposition of magnesium hydroxide decreases the energetic balance of the material under combustion and dilutes the volatile products of composite destruction decreasing the concentration of flammable gases [27]. Magnesium oxide resulted from the decomposition of Mg(OH)2 facilitates carbonization processes on the surface of the material under combustion (Fig. 14b, d).
It should be clearly emphasized that the flame-retardant compounds used also decrease the toxicity of gaseous products formed during the thermal decomposition and combustion of the elastomeric materials investigated.
Table 8 shows the emission of six most harmful gases for living organisms occurring during the decomposition of L800/NBR30 blend as well as L800/NBR30/CM50 and L800/NBR30/Mg80 composites. It should be stated that in the presence of halogenless flame-retardant compounds, the emission of toxic CO and HCN was considerably decreased in relations to L800/NBR30 sample.
Undoubtedly, the reduced emission of these gases results first of all from the emission of biologically neutral gases, e.g., water vapor.
It should be also noticed that as a result of the combustion of the composite filled with melamine cyanurate (this compound contains 60–70 % by wt. of nitrogen), the specific emission of NO2 increased to a very small extent.
Table 9 shows the values of toxicometric parameters. The value of W LC50SM according to standard PN-88/B-02855 is the classification basis of the products of thermal decomposition and combustion into the following groups:
Based on the above classification, the products formed during the decomposition of L800/NBR30 composites should be rated among toxic products, while those resulted from the decomposition of composites filled with the flame retardants used belong to moderately toxic products.
It should be underlined that the laboratory test results fail to completely reflect the real hazard occurring under natural conditions during fire. However, they allow one to perform a comparative analysis of the combustion results of various materials under the same conditions, which gives the basis for the assessment which of the material tested, constitutes greater hazard for man and the environment during fire.
Mechanical properties
Practically, selecting flame-retardant compounds, one should take into account a distinct reduction in the flammability of polymeric materials, maintaining or improving their physical and mechanical parameters at the same time.
From the data listed in Table 10, it follows that melamine cyanurate slightly deteriorates the mechanical properties of the composites obtained. On the other hand, the functional properties of the materials obtained are improved under the influence of magnesium hydroxide. It is not without significance that the processing of elastomeric blends containing the flame retardants on a roll stand is also considerably easier.
Conclusions
The thermal properties of cross-linked EVM/NBR blends depend on the content of NBR rubber. An increase in the NBR content in the composites investigated increases their temperature of the beginning of thermal decomposition and considerably decreases the rate of the first thermal decomposition stage, dm/dt I.
The incorporation of conventional halogenless flame retardants into L450/NBR30 and L800/NBR30 blends significantly improves the thermal stability of the polymeric materials obtained. This contributes to a considerable reduction in their flammability and makes it possible to obtain self-extinguishing or nonflammable composites.
Taking into account the quantity of flame retardant incorporated into the blends and fire hazard parameters, such as the THR, HRR, and mass loss rate, it should be stated that melamine cyanurate shows a similar effectiveness to that of magnesium hydroxide.
The gaseous products of the thermal decomposition and combustion of L800/NBR30 containing no flame retardant are rated among toxic compounds, while those resulted from the thermal decomposition of the composite filled with flame retardants are classified to the group of moderately toxic products.
References
Purser DA, Woolley WD. Biological studies of combustion atmosphere. J Fire Sci. 1983;1:118–44.
Purser DA. Behavioural impairment in smoke environments. Toxicology. 1996;115:25–40.
Hartzell GE. Overview of combustion toxicology. Toxicology. 1996;115:7–23.
Smith SM, Stuhmiller JH, Januszkiewicz AJ. Evaluation of lethality estimates for combustion gases in military scenarios. Toxicology. 1996;115:157–65.
Janowska G, Kucharska-Jastrząbek A, Rybiński P. Flammability of diene rubbers. J Therm Anal Calorim. 2010;102:1043–9.
Rybiński P, Janowska G, Jóżwiak M, Pająk A. Thermal properties and flammability of nanocomposites based on diene rubbers and naturally occuring and activated halloysite nanotubes. J Therm Anal Calorim. 2012;107:1243–9.
Troitzsch J. International plastics flammability handbook. 2nd ed. Munich: Hanser Publishers; 1990.
Rybiński P, Janowska G, Kucharska-Jastrząbek A, Pająk A, Wójcik I, Wesołek D, Bujnowicz K. Flammability of vulcanizates of diene rubbers. J Therm Anal Calorim. 2012;107:1219–24.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Flynn JH, Wall LA. A quick, direct method for determination of activation energy from thermogravimetric data. J Polym Sci Polym Lett. 1966;4:323–8.
Cheng HKF, Saho NG, Lu X, Li L. Thermal kinetics of montmorillonite nanoclay/maleic anhydride-modified polypropylene nanocomposites. J Therm Anal Calorim. 2012;109:17–25.
Ak M, Cilgi GK, Kuru FD, Cetisli H. Thermal decomposition kinetics of polypyrrole and its star shaped copolymer. J Therm Anal Calorim. 2013;111:1627–32.
Maiti M, Mitra S, Bhowmick AK. Effect of nanoclays on high and low temperature degradation of fluoroelastomers. Polym Degrad Stab. 2008;93:188–200.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29(11):1702–6.
Rybiński P, Janowska G, Jóźwiak M. Thermal stability and flammability of butadiene-styrene rubber nanocomposites. J Therm Anal Calorim. 2012;109:561–71.
Rybiński P, Janowska G. Thermal properties and flammability of nanocomposites based on nitrile rubbers and activated halloysite nanotubes and carbon nanofibers. Thermochim Acta. 2012;549:6–12.
Grażyna J, Rybiński P, Krauze S. The effect of curing agent on the flammability of butadiene-acrylonitrile rubbers. Polimery. 2006;10(51):735–41.
Rybiński P, Janowska G. Effect of flame retardants on thermal stability and flammability of cured nitrile rubber. Polimery. 2009;11–12(54):833–9.
Kaufmann S (1994) In: Bloor D, Brook RJ, Flemings MC, Mahajan S (eds) Encyclopedia of advanced materials, vol 2. Pergamon Press, London, p 851
Janowska G, Przygocki W, Włochowicz A. Palność polimerów i materiałów polimerowych. Warszawa: WNT; 2007.
Liu H, Xiong Y, Xu W, Zhang Y, Pan S. Synthesis of a novel intumescent flame retardant and its application in EVM. J Appl Polym Sci. 2012;125:1544–51.
Li Z, Qu B. Effects of gamma irradiation on the properties of flame-retardant EVM/magnesium hydroxide blends. Radiat Phys Chem. 2004;69:137–41.
Wang J, Cai X. Kinetics study of thermal oxidative degradation of ABS containing flame retardant components. J Therm Anal Calorim. 2012;107:725–32.
Yuan D, Yin H, Cai X. Effect of a novel flame retardant containing silicon and nitrogen on the thermal stability and flame retardancy of polycarbonate. J Therm Anal Calorim. 2013;111:1531–7.
Rybiński P, Janowska G. Influence synergetic effect of halloysite nanotubes and halogen flame-retardants on properties nitrile rubber composites. Thermochim Acta. 2013;557:24–30.
Thirumal M, Nando GB, Naik YP, Nikhil KS. Halogen-free flame retardant PUF: effect of melamine compounds on mechanical thermal and flame retardant properties. Polym Degrad Stab. 2010;95:1138–45.
Yeh J, Yan M, Hsieh S. Combustion of polyethylenes filled with metallic hydroxides and ethylene vinyl acetate copolymer. Polym Degrad Stab. 1998;61:465–72.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Rybiński, P., Janowska, G., Dobrzyńska, R. et al. Effect of halogenless flame retardants on the thermal properties, flammability, and fire hazard of cross-linked EVM/NBR rubber blends. J Therm Anal Calorim 115, 771–782 (2014). https://doi.org/10.1007/s10973-013-3333-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3333-7