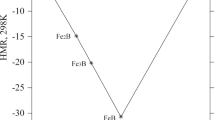
Abstract
Uranium–zirconium, uranium niobium, and uranium–zirconium–niobium alloys were synthesized by the arc melting technique and their phase transition temperatures were determined using a high temperature calorimeter. Heat capacities of U–7 wt%Zr, U–7 wt%Nb, U–5 wt%Zr–2 wt%Nb, U–3.5 wt%Nb–3.5 wt%Zr, and U–2 wt%Zr–5 wt%Nb were measured using a differential scanning calorimeter in the temperature range 303–921 K. A set of self-consistent thermodynamic functions such as entropy, enthalpy, and Gibbs energy function data for these binary and ternary alloys were reported for the first time using heat capacity data obtained in this study and required literature data.





Similar content being viewed by others
References
Lee CB, Mizuno T, Delage F, Somers J. Metallic fuels for advanced reactors. J Nucl Mater. 2009;392:139–50.
Riyas A, Mohanakrishnan P. Studies on physics parameters of metal (U–Pu–Zr) fuelled FBR cores. Ann Nucl Energy. 2008;35:87–92.
Meyer MK, Hofman GL, Wiencek TC, Hayes SL, Snelgrove JL. Irradiation behavior of U–Nb–Zr alloy dispersed in aluminum. J Nucl Mater. 2001;299:175–9.
Heraud FG, Guillaumint J. Transition phase formation in U–7.5%Nb–2.5 Zr alloy. Acta Metallur. 1973;21:1243–52.
Savchenko A, Vatulin A, Konovalov I, Morozov A, Sorokin V, Maranchak S. Fuel of novel generation for PWR and as alternative to MOX fue. Energy Convers Manage. 2010;51:1826–33.
Lagerberg G. Phase transformations in a uranium–zirconium alloy containing 2 weight per cent zirconium. J Nucl Mater. 1963;9:261–76.
Ogawa T, Ogata T, Itoh A, Akabori M, Miyanishi H, Sekino H, Nishi M, Shikawa A. Irradiation behavior of microspheres of U–Zr alloys. J Alloy Compd. 1998;12:670–5.
Sheldon RI, Peterson DE. U–Zr phase diagram. In: Massalski TB, editor. Binary alloy phase diagrams, vol II. Metals Park: American Society of Metals, 1986. p 2150.
Koike J, Kassner ME, Tate RE, Rosen RS. The Nb–U (niobium–uranium) system. J Phase Equilib. 1998;19:253–60.
Dwight AE, Mueller MH. Constitution of the uranium-rich U–Nb and U–Nb–Zr systems. Argonne National Laboratory Report, ANL-5581; 1957.
Peterson CAW, Vandervoort R. Recent observations of phase transformations in a U–Nb–Zr alloy. University of California, Lawrence Radiation Laboratory, Livermore Report UCRL-7869; 1964.
Ghoshal K, Kutty TRG, Mishra S, Kumar A. Creep studies on U–7%Zr, U–7%Nb and U rich U–Nb–Zr alloys. J Nucl Mater. 2013;432:20–2.
Williams RO. Stability of the body-centered cubic gamma phase in the uranium–zirconium-niobium system. J Nucl Mater. 1979;82:184–92.
Villars P, Prince A, Okamoto H. Hand book of ternary alloy system, vol 10. Materials Park: ASM International, The Materials Information Society. 2009.
Dash S, Parida SC, Singh Z, Sen BK, Venugopal V. Thermodynamic investigations of ThO2–UO2 solid solutions. J Nucl Mater. 2009;393:267–81.
Parida SC, Rakshit SK, Dash S, Singh Z, Sen BK, Venugopal V. Systems R–Fe–O (R = Ho, Er): thermodynamic properties of ternary oxides using differential scanning calorimetry and solid-state electrochemical cells. J Solid State Chem. 2006;179:2212–30.
Rough FA. An evaluation on zirconium–uranium alloys, report no. BMI-1030. 16th ed. Metallurgy and ceramics (M-3679). Columbus: Battelle Memorial Institute; 1955.
Bauer AA. An evaluation of the properties and behaviour of zirconium–uranium alloys, report no. BMI-1350. 15th ed. Metallurgy and ceramics (TID-4500). Columbus: Battelle Memorial Institute; 1959.
Fedorov GB, Smirnov EA. Heat capacity of uranium–zirconium systems. Sov At Energy. 1968;25:795–7.
Takahashi Y, Yamawaki M, Yamamoto K. Thermophysical properties of uranium–zirconium alloys. J Nucl Mater. 1988;154:141–4.
Takahashi Y, Yamamoto K, Ohsato T, Shimada H, Terai T, Yamawaki M. Heat capacities of uranium–zirconium alloys from 300 to 1100 K. J Nucl Mater. 1989;167:147–51.
Matsui T, Natsume T, Naito K. Heat capacity measurements of U0.80Zr0.20 and U0.80Mo0.20 alloys from room temperature to 1300 K. J Nucl Mater. 1989;167:152–9.
Kaity S, Banerjee J, Nair MR, Ravi K, Dash S, Kutty TRG, Kumar A, Singh RP. Microstructural and thermophysical properties of U–6 wt%Zr alloy for fast reactor application. J Nucl Mater. 2012;427:1–11.
Vambersky YuV, Udovsky AL, Ivanov OS. Investigation of thermodynamic properties of BCC solid solutions of uranium (II). The uranium–niobium system. J Nucl Mater. 1975;55:96–108.
Zhang X, Wang X, Luo C. Thermodynamic calculation of U-based binary melts. Rare Met Mater Eng. 2009;38:603–6.
Drotning WD. Density and thermal expansion of liquid U–Nb alloys. High Temp High Press. 1982;14:253–8.
Barin I. Thermochemical data of pure substances. Weinheim (Federal Republic of Germany): Verlagsgesellschaft mbH; 1995.
Acknowledgements
The authors are thankful to Shri S.G. Kulkarni, Head, the Product Development Division, and Shri Arun Kumar, Head, the Radiometallurgy Division, and Dr. K.L Ramakumar, Director, the Radiochemistry and Isotope Group, for their keen interest in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dash, S., Ghoshal, K. & Kutty, T.R.G. Thermodynamic investigations of uranium-rich binary and ternary alloys. J Therm Anal Calorim 112, 179–185 (2013). https://doi.org/10.1007/s10973-012-2801-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2801-9