Abstract
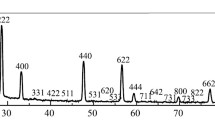
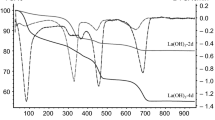
Lanthanum oxide (La2O3) is of great interest as catalyst material. When La2O3 particles are prepared from lanthanum hydroxide (La(OH)3) by thermal processes under air, various oxycarbonate phases are formed which are resistant to thermal hydroxylation. This phenomenon has not yet been extensively investigated, even though oxycarbonate phases at the particle surfaces cause a change in lanthanum oxide’s catalytic activity. The carbonate phases formed cannot be detected by means of XRD or REM-EDX investigations due to their detection limits. Thermal analysis, particularly TG-FT-IR, allows not only for the detection of the carbonate phases in La(OH)3, but also for the tracking of the entire dehydration process from La(OH)3 via LaOOH to La2O3 as well as the correct interpretation of mass changes during the thermal transformations. Pursuant to the investigations here carried out, it was determined that carbonate-free lanthanum hydroxide compounds can only be prepared and stored in a CO2-free protective gas atmosphere (e.g., argon).






Similar content being viewed by others
References
Barnard KR, Foger K, Turney TW, Williams RD. Lanthanum cobalt oxide oxidation catalysts derived from mixed hydroxide precursors. J Catal. 1990;125:265–75.
Vishnyakov AV, Korshunova IA, Kochurikhin VE, Sal’nikova LS. Catalytic activity of rare earth oxides in flameless methane combustion. Kinet Catal. 2010;51:273–8.
Sampath S, Kulkarni NK, Subramanian MS, Jayadevan NC. Effect of lanthanum, neodymium, thorium, uranium, and plutonium compounds on graphite oxidation. Carbon. 1988;26:129–37.
Li SL, Zhang SX, Hu H, Zhang YH. Influence of pulse discharge plasma on composition of La2O3 catalyst. Chin J Catal. 2004;25:762–6.
Lin CH, Campbell KD, Wang JX, Lunsford JH. Oxidative dimerization of methane over lanthanum oxide. J Phys Chem. 1986;90:534–7.
Wan HL, Zhou XP, Weng WZ, Long RQ, Chao ZS, Zhang WD, Chen MS, Luo JZ, Zhou SQ. Catalytic performance, structure, surface properties and active oxygen species of the fluoride-containing rare earth (alkaline earth)-based catalysts for the oxidative coupling of methane and oxidative dehydrogenation of light alkanes. Catal Today. 1999;51:161–75.
Ertl G, Knözinger H. Handbook of heterogeneous catalysis. Weinheim: Wiley; 1997.
Walter D. Kinetic analysis of the transformation from lanthanum hydroxide to lanthanum oxide. Z Anorg Allg Chem. 2006;632:2165.
Christensen AN. Hydrothermal preparation and low temperature magnetic properties of TbOOH, DyOOH, HoOOH, ErOOH, and YbOOH. J Solid State Chem. 1972;4:46–51.
Neumann A, Walter D. The thermal transformation from lanthanum hydroxide to lanthanum hydroxide oxide. Thermochim Acta. 2006;445:200–4.
Fleming P, Farrell RA, Holmes JD, Morris MA. The rapid formation of La(OH)3 from La2O3 powders on exposure to water vapor. J Am Ceram Soc. 2010;93:1187–94.
Bernal S, Botana FJ, Garcia R, Rodriguez-Izquierdo JM. Thermal evolution of a sample of La2O3 exposed to the atmosphere. Thermochim Acta. 1983;66:139–45.
Larson AC, von Dreele RB. GSAS 1994 Version 2000. Vol. 86. Los Alamos National Laboratory Report (LAUR); 2000.
Yamamoto O, Takeda Y, Kanno R, Fushimi M. Thermal-decomposition and electrical-conductivity of M(OH)3 and MOOH (M = Y, Lanthanide). Solid State Ionics. 1985;17:107–14.
Beall GW, Milligan WO, Wolcott HA. Structural trends in lanthanide trihydroxides. J Inorg Nucl Chem. 1977;39:65–70.
Acknowledgements
We would like to thank Dr. Anja Neumann and M.Sc. Elena Haibel for providing sample material and helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Füglein, E., Walter, D. Thermal analysis of lanthanum hydroxide. J Therm Anal Calorim 110, 199–202 (2012). https://doi.org/10.1007/s10973-012-2298-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2298-2