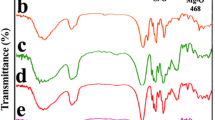
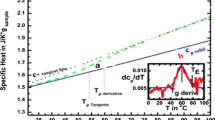
Abstract
Nanocomposites of poly(l-lactic acid) (PLLA) containing 2.5 wt% of fumed silica nanoparticles (SiO2) and organically modified montmorillonite (OMMT) were prepared by solved evaporation method. From SEM micrographs it was observed that both nanoparticles were well dispersed into PLLA matrix. All nanocomposites exhibited higher mechanical properties compared to neat PLLA, except elongation at break, indicating that nanoparticles can act as efficient reinforcing agents. Nanoparticles affect, also, the thermal properties of PLLA and especially the crystallization rate, which in all nanocomposites is faster than that of neat PLLA. From the thermogravimetric curves it can be seen that neat PLLA nanocomposites present a relatively better thermostability than PLLA, and this was also verified from the calculation of activation energy (E). From the variation of E with increasing degree of conversion it was found that PLLA/nanocomposites decomposition takes place with a complex reaction mechanism, with the participation of two different mechanisms. The combination of models, nth order and nth order with autocatalysis (Fn–Cn), for PLLA and PLLA/OMMT as well as the combination of Fn–Fn for PLLA/SiO2 give the better results. For the PLLA/OMMT the values of the E for both mechanisms are higher than neat PLLA. For the PLLA/SiO2 nanocomposite the value of the E is higher than the corresponding value for PLLA, for the first area of mass loss, while the E of the second mechanism has a lower value.









Similar content being viewed by others
References
Hayashi T. Biodegradable polymers for biomedical uses. Prog Polym Sci. 1994;19:663–702.
Rezwan K, Chen QZ, Blaker JJ, Boccaccini AR. Biodegradable, bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27:3413–37.
Balasundaram G, Webster TJ. An overview of nano-polymers for orthopedic applications. Macromol Biosci. 2007;7:635–42.
Boccaccini AR, Gerhardt LS, Rebeling S, Blaker JJ. Fabrication, characterization and assessment of bioactivity of poly(d/l-lactic acid) (PDLLA)/TiO2 nanocomposite films. Compos A. 2005;36:721–7.
Abarrategi A, Gutierrez M, Moreno-Viccente C, Hortiguala MJ, Ramos V, Lopez-Lacomba JL, Ferrer M, Del Monte F. Multiwall carbon nanotube scaffold for tissue engineering purposes. Biomaterials. 2008;29:94–102.
Kotela I, Podporska J, Soltysiak E, Konsztowicz KJ, Blazewicz M. Polymer nanocomposites for bone tissue substitutes. Ceram Int. 2009;35:2475–80.
Agrawal M, Ray RB. Biodegradable polymeric scaffolds for musculoskeletal tissue engineering. J Biomed Mater Res. 2001;55:141–50.
Wei G, Ma PX. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials. 2004;25:4749–57.
Satyanarayana D, Chatterji PR. Biodegradable polymers: challenges and strategies. J Macromol Sci Rev Macromol Chem Phys. 1993;C33:39–368.
Cheung HY, Lau KT, Lu TP, Hui D. A critical review on polymer-based bio-engineered materials for scaffold development. Compos B Eng. 2007;38:291–300.
Bleach NC, Nazhat SN, Tanner KE, Kellomäki M, Törmälä P. Effect of filler content on mechanical and dynamic mechanical properties of particulate biphasic calcium phosphate—polylactide composites. Biomaterials. 2002;23:1579–85.
Alexander H, Langrana N, Massengill J, Weiss A. Development of new methods for phalangeal fracture fixation. J Biomech. 1981;14:377–87.
Vassiliou A, Papageorgiou GZ, Achilias DS, Bikiaris DN. Non-isothermal crystallization kinetics of in situ prepared poly(ε-caprolactone)/surface-treated SiO2 nanocomposites. Macromol Chem Phys. 2007;21:364–76.
Vassiliou A, Chrissafis K, Bikiaris DN. In situ prepared PBSu/SiO2 nanocomposites. Study of thermal degradation mechanism. Thermochim Acta. 2009;495:120–8.
Yan S, Yin J, Yang Y, Dai Z, Ma J, Chen X. Surface-grafted silica linked with l-lactic acid oligomer: a novel nanofiller to improve the performance of biodegradable poly(l-lactide). Polymer. 2007;48:1688–94.
Wu CS, Liao HT. Modification of biodegradable polylactide by silica and wood flour through a sol-gel process. J Appl Polym Sci. 2008;109:2128–38.
Wu L, Cao D, Huang Y, Li B. Poly(l-lactic acid)/SiO2 nanocomposites via in situ melt polycondensation of l-lactic acid in the presence of acidic silica sol: preparation and characterization. Polymer. 2008;49:742–9.
Zhou Q, Xanthos M. Nanosize and microsize clay effects on the kinetics of the thermal degradation of polylactides. Polym Degrad Stab. 2009;94:327–38.
Jiang L, Zhang J, Wolcott MP. Comparison of polylactide/nano-sized calcium carbonate and polylactide/montmorillonite composites: reinforcing effects and toughening mechanisms. Polymer. 2007;48:7632–44.
Wen X, Lin Y, Han C, Zhang K, Ran X, Li Y, Dong L. Thermomechanical and optical properties of biodegradable poly(l-lactide)/silica nanocomposites by melt compounding. J Appl Polym Sci. 2009;114:3379–86.
Chang JH, An YA, Sur GS. Poly(lactic acid) nanocomposites with various organoclays. I. Thermomechanical properties, morphology, and gas permeability. J Polym Sci B Polym Phys. 2003;41:94–103.
Migliaresi CD, Cohn D, De Lollis A, Fambri L. Dynamic mechanical and calorimetric analysis of compression-molded PLLA of different molecular weights. Effect of thermal treatments. J Appl Polym Sci. 1991;43:83–95.
Gopakumar TG, Lee JA, Kontopoulou M, Parent JS. Influence of clay exfoliation on the physical properties of montmorillonite/polyethylene composites. Polymer. 2002;43:5483–91.
Antoniadis G, Paraskevopoulos KM, Bikiaris D, Chrissafis K. Kinetics study of cold-crystallization of poly(ethylene terephthalate) nanocomposites with multi-walled carbon nanotubes. Thermochim Acta. 2009;493:68–75.
Di YW, Iannace S, Di ME, Nicolais L. Poly(lactic acid)/organoclay nanocomposites: thermal, rheological properties and foam processing. J Polym Sci B Polym Phys. 2005;43:689–98.
Chrissafis K, Paraskevopoulos KM, Papageorgiou GZ, Bikiaris DN. Thermal and dynamic mechanical behavior of bionanocomposites: fumed silica nanoparticles dispersed in poly(vinyl pyrrolidone), chitosan, and poly(vinyl alcohol). J Appl Polym Sci. 2008;110:1739–49.
Chen K, Wilkie CA, Vyazovkin S. Nanoconfinement revealed in degradation and relaxation studies of two structurally different polystyrene-clay systems. J Phys Chem B. 2007;111:12685–12692.
Yoon JT, Jeong YG, Lee SC, Min BG. Influences of poly(lactic acid)-grafted carbon nanotube on thermal, mechanical, and electrical properties of poly(lactic acid). Polym Adv Technol. 2009;20:631–8.
Krishnamachari P, Zhang J, Lou J, Yan J, Uitenham L. Biodegradable poly(lactic Acid)/clay nanocomposites by melt intercalation: a study of morphological, thermal, and mechanical properties. Int J Polym Anal Charact. 2009;14:336–50.
Chow WS, Lok SK. Thermal properties of poly(lactic acid)/organo-montmorillonite nanocomposites. J Therm Anal Calorim. 2009;95:627–32.
Zhang J, Lou J, Ilias S, Krishnamachari P, Yan J. Thermal properties of poly(lactic acid) fumed silica nanocomposites: experiments and molecular dynamics simulations. Polymer. 2008;49:2381–6.
McLauchlin AR, Thomas NL. Preparation and thermal characterization of poly(lactic acid) nanocomposites prepared from organoclays based on an amphoteric surfactant. Polym Degrad Stab. 2009;94:868–72.
Chang JH, An YU, Cho D, Giannelis EP. Poly(lactic acid) nanocomposites: comparison of their properties with montmorillonite and synthetic mica. Polymer. 2003;44:3715–20.
Vyazovkin S. Model-free kinetics. Staying free of multiplying entities without necessity. J Therm Anal Calorim. 2007;83:45–51.
Starink MJ. On the applicability of isoconversion methods for obtaining the activation energy of reactions within a temperature-dependent equilibrium state. J Mater Sci. 1997;32:6505–12.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Flynn JH, Wall LA. General treatment of the thermogravimetry of polymers. J Res Natl Bur Stand Phys Chem. 1966;70A:487–523.
Flynn JH, Wall LA. A quick, direct method for the determination of activation energy from thermogravimetric data. Polym Lett. 1966;4:323–8.
Friedman HL. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J Polym Sci C. 1964;6:183–95.
Vyazovkin S. Modification of the integral isoconversional method to account for variation in the activation energy. J Comput Chem. 2001;22:178–83.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–08.
Zou H, Yi C, Wang L, Liu H, Xu W. Thermal degradation of poly(lactic acid) measured by thermogravimetry coupled to Fourier transform infrared spectroscopy. J Therm Anal Calorim. 2009;97:929–35.
Opfermann J. Kinetic analysis using multivariate non-linear regression i. Basic concepts. J Therm Anal Calorim. 2000;60:641–58.
Chrissafis K, Paraskevopoulos KM, Jannakoudakis A, Beslikas T, Bikiaris D. Oxidized multi-walled carbon nanotubes as effective reinforcement and thermal stability agents of PLLA ligaments. J Appl Polym Sci. 2010;118:2712–21.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chrissafis, K., Pavlidou, E., Paraskevopoulos, K.M. et al. Enhancing mechanical and thermal properties of PLLA ligaments with fumed silica nanoparticles and montmorillonite. J Therm Anal Calorim 105, 313–323 (2011). https://doi.org/10.1007/s10973-010-1168-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-1168-z