Abstract
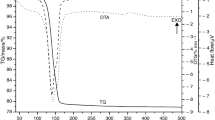
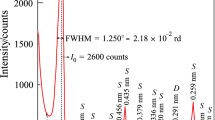
The dehydration behaviors of FGD gypsums from three power plants were investigated at N2 atmosphere (autogenous and negligible partial pressure of water, \( P_{{{\text{H}}_{ 2} {\text{O}}}} \)) in non-isothermal and isothermal condition. The dehydration of gypsum proceeded through one step, i.e., CaSO4·2H2O → γ-CaSO4 (γ-anhydrite) or two steps, i.e., CaSO4·2H2O → CaSO4·0.5H2O (hemihydrate) → γ-CaSO4 depending on temperature and \( P_{{{\text{H}}_{ 2} {\text{O}}}} \). The discrepancies of three FGD gypsums on dehydration behavior were very likely due to the different crystalline characteristics (size and habit) and impurities, such as fly ash and limestone. Experimental data of non-isothermal analysis have been fitted with two ‘model-free’ kinetic methods and those of isothermal analysis have been fitted with Avrami and linear equation. The apparent empirical activation energies (E a) suggest that the transition from gypsum to hemihydrate is mainly controlled by nucleation and growth mechanism, while the transition from gypsum to γ-anhydrite is mostly followed by phase boundary mechanism.









Similar content being viewed by others
References
Freyer D, Voigt W. Crystallization and phase stability of CaSO4 and CaSO4-based salts. Monatsh Chem. 2003;134:693–719.
Solberg C, Evju C, Emanuelson A, Hansen S. Crystal structures of cementitious compounds. Part 3: calcium sulfates. ZKG Int. 2002;55:94–7.
Christensen AN, Olesen M, Cerenius Y, Jensen TR. Formation and transformation of five different phases in the CaSO4·H2O system: crystal structure of subhydrate β-CaSO4·0.5H2O and soluble anhydrite CaSO4. Chem Mater. 2008;20:2124–32.
Charola AE, Pűhringer J, Steiger M. Gypsum: a review of its role in the deterioration of building materials. Environ Geol. 2007;52:339–52.
Ballirano P, Melis E. Thermal behaviour and kinetics of dehydration of gypsum in air from in situ real-time laboratory parallel-beam X-ray powder diffraction. Phys Chem Mineral 2009;36:391–402.
McAdie HG. The effect of water vapor upon the dehydration of CaSO4·2H2O. Can J Chem. 1964;42:792–801.
Bushuev NN, Maslennikov BM, Borisov VM. X-ray diffraction investigation of CaSO4·0.67H2O. Russ J Inorg Chem. 1982;27:341–3.
Christensen AN, Lehmann MS, Pannetier J. A time-resolved neutron powder diffraction investigation of the hydration of CaSO4·1/2D2O and of the dehydration of CaSO4·2D2O. J Appl Cryst. 1985;18:170–2.
Abriel W, Reisdorf K, Pannetier J. Dehydration reactions of gypsum: a neutron and X-ray diffraction study. J Solid State Chem. 1990;85:23–30.
Putnis A, Winkler B. In situ IR spectroscopic and thermogravimetric study of the dehydration of gypsum. Mineral Mag. 1990;54:123–8.
Strydom CA, Hudson-Lamb DL, Potgieter JH, Dagg E. The thermal dehydration of synthetic gypsum. Thermochim Acta. 1995;269(/270):631–8.
Dos Santos VA, Pereira JAFR, Dantas CC. Kinetics of thermal dehydration of gypsum ore for obtaining beta hemihydrate in a fluidized bed. Bull Soc Chim Belg. 1997;6:253–60.
Chang H, Huang PJ, Hou SC. Application of thermo-Raman spectroscopy to study dehydration of CaSO4·2H2O and CaSO4·0.5H2O. Mater Chem Phys. 1999;58:12–9.
Carbone M, Ballirano P, Caminiti R. Kinetics of gypsum dehydration at reduced pressure: an energy dispersive X-ray diffraction study. Eur J Mineral. 2008;20:621–7.
Molony B, Ridge MJ. Kinetics of the dehydration of calcium sulphate dihydrate in vacuo. Aust J Chem. 1968;21:1063–5.
Sarma LP, Prasad PSR, Ravikumar N. Raman spectroscopic study of phase transitions in natural gypsum. J Raman Spectrosc. 1998;29:851–6.
Prasad PSR, Pradhan A, Gowd TN. In situ micro-Raman investigation of dehydration mechanism in natural gypsum. Curr Sci. 2001;80:1203–7.
Chio CH, Sharma SK, Muenow DW. Micro-Raman studies of gypsum in the temperature range between 9 K and 373 K. Am Mineral. 2004;89:390–5.
Prasad PSR, Chaitanya VK, Prasad KS, Rao DN. Direct formation of the γ-CaSO4 phase in dehydration process of gypsum: in situ FTIR study. Am Mineral. 2005;90:672–8.
Ball MC, Norwood LS. Studies in the system calcium sulphate-water. Part I. Kinetics of dehydration of calcium sulphate dihydrate. J Chem Soc A. 1969;1633–7.
Badens E, Llewellyn P, Fulconis JM, Jourdan Veesler CS, Boistelle R, et al. Study of gypsum dehydration by controlled transformation rate thermal analysis (CRTA). J Solid State Chem. 1998;139:37–44.
Fatu D. Kinetics of gypsum dehydration. J Therm Anal Calorim. 2001;65:213–20.
Hudson-Lamb DL, Strydom CA, Potgieter JH. The thermal dehydration of natural gypsum and pure calcium sulphate dehydrate (gypsum). Thermochim Acta. 1996;282(/283):483–92.
Jordan G, Astilleros JM. In situ HAFM study of the thermal dehydration on gypsum (010) surfaces. Am Mineral. 2006;91:619–27.
Strydom CA, Potgieter JH. Dehydration behaviour of a natural gypsum and a phosphogypsum during milling. Thermochim Acta. 1999;332:89–96.
Cave SR, Holdich RG. The dehydration kinetics of gypsum in a fluidized bed reactor. Chem Eng Res Des. 2000;78:971–8.
Deutsch Y, Nathan Y, Sarig S. Thermogravimetric evaluation of the kinetics of the gypsum-hemihydrate-soluble anhydrite transitions. J Therm Anal Calorim. 1994;42:159–74.
Hamm H, Kersten HJ, Hueller R. 25 years experience gained in the European Gypsum Industry with the use of FGD gypsum. CEM Int. 2004;4:92–102.
Guan B, Yang L, Wu Z, Shen Z, Ma X, Ye Q. Preparation of α-calcium sulfate hemihydrate from FGD gypsum in K, Mg-containing concentrated CaCl2 solution under mild conditions. Fuel. 2009;88:1286–93.
Follner S, Wolter A, Helming K, Silber C, Bartels H, Follner H. On the real structure of gypsum crystals. Cryst Res Tech. 2002;37:207–18.
Brown ME, Maciejewski M, Vyazovkin S, Nomen R, Sempere J, Burnham A, et al. Computational aspects of kinetic analysis. Part A: the ICTAC kinetics project-data, methods and results. Thermochim Acta. 2000;355:125–43.
Farjas J, Butchosa N, Roura P. A simple kinetic method for the determination of the reaction model from non-isothermal experiments. J Therm Anal Calorim. doi:10.1007/s10973-010-0737-5.
Acknowledgements
The authors acknowledge greatly the financial support of this work by the fund of Chinese National Program for High Technology Research and Development (Project No. 2006AA03Z385), Science and Technology Plan of Zhejiang Province, China (Project No. 2007C23055).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lou, W., Guan, B. & Wu, Z. Dehydration behavior of FGD gypsum by simultaneous TG and DSC analysis. J Therm Anal Calorim 104, 661–669 (2011). https://doi.org/10.1007/s10973-010-1100-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-1100-6