Abstract
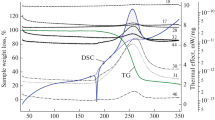
Polyaniline was obtained by oxidizing aniline in hydrochloric acid media with ammonium peroxidisulfate as oxidizing agent. Molar ratio aniline/oxidant was 1 and aniline/acid ratio: ½, at −5 and 400 °C, respectively, 800 mL water. The both compounds were studied using two different experimental strategies: the coupled TG-EGA (FTIR) technique by decomposition in dynamic air atmosphere and the kinetic analysis of TG data obtained at four heating rates (5, 7, 10 and 15 K min−1). The kinetic analysis of the TG non-isothermal data was performed with the Flynn–Wall–Ozawa, Friedman’s, and modified non-parametric kinetic (NPK) methods. By means of the coupled techniques spectroscopic arguments on the reaction mechanism were obtained, i.e. the oxidative degradation of the quinoine ring as the first step. The values of the activation energy by the three used methods are in good agreements. According to the NPK method, the termodegradation process consist in physical (diffusion) and chemical steps.







Similar content being viewed by others
References
Nalwa HS. Handbook of conductive molecules and polymers. London: Wiley; 1997. 2503 pp.
MacDiarmid AG, Chiang JC, Richter AF. Polyaniline: a new concept in conducting polymers. Synth Met. 1987;18:285–90.
Hatchett DW, Josowicz M, Janata J. Acid doping of polyaniline: spectroscopic and electrochemical studies. J Phys Chem B. 1999;103:10992–8.
Wei Y, Yeh JM, Iin D, Iia X, Wang J, Jang G-W, et al. Composites of electronically conductive polyaniline with polyacrylate-silica hybrid sol-gel materials. Chem Mater. 1995;7:969–74.
Mattes BR, Knoble ET, Fuqua PD, Nishida F, Chang E-W, Dunn B, et al. Polyaniline sol-gels and their third-order nonlinear optical effects. Synth Met. 1991;41:3183–7.
Asturias GE, MacDiarmid AG, McCall RP, Epstein AJ. The oxidation state of “emeraldine” base. Synth Met. 1989;29:157–62.
Shacklette LW, Wolf JF, Gould S, Baughman RH. Structure and properties of polyaniline as modeled by single-crystal oligomers. J Chem Phys. 1988;88:3955–61.
Chen CH. Thermal and morphological studies of chemically prepared emeraldine-base-form polyaniline powder. J Appl Polym Sci. 2003;89:2142–8.
Trchova M, Sedenkova I, Tobolkova E, Stejskal J. FTIR spectroscopic and conductivity study of the thermal degradation of polyaniline films. Polym Degrad Stab. 2004;86:179–85.
Palaniappan S, Devi SL. Thermal stability and structure of electroactive polyaniline–fluoroboric acid–dodecylhydrogensulfate salt. Polym Degrad Stab. 2006;91:2415–22.
Brown ME, Maciejewski M, Vyazovkin S, Nomen R, Sempere J, Burnham A, et al. Computational aspects of kinetic analysis: part A: the ICTAC kinetics project-data, methods and results. Thermochim Acta. 2000;355:125–43.
Flynn IH, Wall LA. A quick, direct method for the determination of activation energy from thermogravimetric data. Polym Lett. 1966;4:323–8.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Friedman HL. Kinetics of thermal degradation of char-foaming plastics from thermogravimetry: application to a phenolic resin. J Polym Sci. 1965;6C:183–95.
Serra R, Nomen R, Sempere J. The non-parametric kinetics a new method for the kinetic study of thermoanalytical data. J Therm Anal Calorim. 1998;52:933–43.
Vlase G, Vlase T, Doca N. Thermal behavior of some phenitoine pharmaceuticals. J Therm Anal Calorim. 2008;92(1):259–62.
Birta N, Doca N, Vlase G, Vlase T. Kinetic of sorbitol decomposition under non-isothermal conditions. J Therm Anal Calorim. 2008;92(2):635–8.
Doca N, Vlase G, Vlase T, Ilia G. Thermal behavior of Cd2+ and Co2+ phenyl-vinyl-phosphonates under non-isothermal condition. J Therm Anal Calorim. 2008;94(2):441–5.
Wall ME. Singular value decomposition and principal component analysis. In: Berrar DP, Dubitzky W, Granzow M, editors. A practical approach to microarray data analysis, vol. 9. LANL LA-UR-02. Norwel, MA: Kluwer; 2003. p. 91–109.
Šesták J, Berggren G. Study of the kinetics of the mechanism of solid-state reactions at increasing temperatures. Thermochim Acta. 1971;3:1–12.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Doca, N., Vlase, G., Vlase, T. et al. TG, EGA and kinetic study by non-isothermal decomposition of a polyaniline with different dispersion degree. J Therm Anal Calorim 97, 479–484 (2009). https://doi.org/10.1007/s10973-009-0217-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0217-y