Abstract
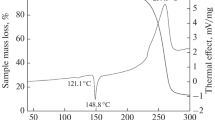
The kinetics of thermal decomposition of 1-[2,2-bis(methoxy-NNO-azoxy)ethyl]-3,4-dinitro-1H-pyrazole is studied using isothermal and nonisothermal methods in a wide temperature range. The composition of the gaseous products, the heat of fusion, and the heat of thermal decomposition are determined. Below the melting temperature, the reaction proceeds with pronounced self-acceleration and cannot be described by simple kinetic laws. The effective activation energies of the process, which decrease as the degree of conversion increases, are calculated. The results obtained are explained from the point of view of general ideas about the mechanism of thermal decomposition of solid organic substances. In the liquid phase, the reaction proceeds with weak self-acceleration. The effective activation energies are also determined for the liquid-phase decomposition. It is assumed that the reaction proceeds according to a consecutive mechanism with the intermediate formation of a cyclic product. It is concluded that the studied 1-[2,2-bis(methoxy-NNO-azoxy)ethyl]-3,4-dinitro-1H-pyrazole is highly thermally stable and that the thermal stability of compounds of this class strongly depends on the number of nitro groups in the pyrazole ring.






Similar content being viewed by others
REFERENCES
I. N. Zyuzin and D. B. Lempert, Russ. Chem. Bull. 34, 753 (1985).
I. N. Zyuzin, G. N. Nechiporenko, N. I. Golovina, et al., Russ. Chem. Bull. 37, 1329 (1997).
I. N. Zyuzin, N. I. Golovina, B. S. Fedorov, et al., Russ. Chem. Bull. 52, 761 (2003).
E. P. Kirpichev, I. N. Zyuzin, V. V. Avdonin, Yu. I. Rubtsov, and D. B. Lempert, Russ. J. Phys. Chem. A 80, 1359 (2006).
I. N. Zyuzin, D. B. Lempert, and G. N. Nechiporenko, Russ. Chem. Bull. 37, 1329 (1988).
I. N. Zyuzin and D. B. Lempert, Russ. J. Gen. Chem. 80, 1792 (2010).
I. N. Zyuzin and D. B. Lempert, Kinet. Catal. 52, 17 (2011).
I. N. Zyuzin and D. B. Lempert, Russ. J. Gen. Chem. 82, 1105 (2012).
I. N. Zyuzin and D. B. Lempert, Russ. J. Gen. Chem. 82, 1891 (2012).
I. N. Zyuzin and D. B. Lempert, Russ. J. Gen. Chem. 84, 831 (2014).
V. V. Zakharov, N. V. Chukanov, I. N. Zyuzin, V. V. Nedel’ko, and B. L. Korsunsii, Russ. J. Phys. Chem. B 13, 62 (2019). https://doi.org/10.1134/S1990793119010305
I. N. Zyuzin, K. Yu. Suponitskii, and I. L. Dalinger, Khim. Geterotsikl. Soedin. 53, 702 (2017).
I. N. Zyuzin, A. I. Kazakov, D. B. Lempert, I. A. Vatsadze, L. S. Kurochkina, and A. V. Nabatova, Combust. Explos., Shock Waves 55, 327 (2019). https://doi.org/10.1134/S0010508219030109
V. V. Zakharov, N. V. Chukanov, G. V. Shilov, G. V. Malkov, A. V. Shastin, and B. L. Korsunskii, Russ. J. Phys. Chem. B 13, 297 (2019). https://doi.org/10.1134/S1990793119020246
B. L. Korsunskii, T. S. Larikova, V. V. Zakharov, V. V. Nedel’ko, N. V. Chukanov, and A. V. Shastin, Russ. J. Phys. Chem. B 13, 632 (2019). https://doi.org/10.1134/S1990793119040201
P. Barret, Cinétique hétérogěnes (Gauthier-Villars, Paris, 1973).
G. B. Manelis and F. I. Dubovitskii, Dokl. Akad. Nauk SSSR 126, 813 (1959).
B. L. Korsunskii and F. I. Dubovitskii, Dokl. Akad. Nauk SSSR 155, 402 (1964).
Funding
This study was carried out on the topic of a state task of Institute of Problems of Chemical Physics, Russian Academy of Sciences, registration number AAAA-A19-119101690058-9 and on the topic of a state task of the Federal Research Center, Semenov Institute of Chemical Physics, registration number AAAA-A17-117040610346-5.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Korsunskiy, B.L., Zakharov, V.V., Larikova, T.S. et al. Thermal Decomposition of 1-[2,2-Bis(Metoxy-NNO-Azoxy)Ethyl]-3,4-Dinitro-1H-Pyrazole. Russ. J. Phys. Chem. B 16, 615–620 (2022). https://doi.org/10.1134/S199079312204008X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S199079312204008X