Abstract
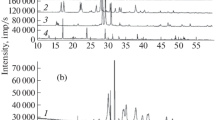
The thermal behavior of Cd2+ and Co2+ phenyl-vinyl-phosphonates was studied using two different experimental strategies: the coupled TG-EGA (FTIR) technique by decomposition in nitrogen respectively air, and the kinetic analysis of TG data obtained in dynamic air atmosphere at four heating rates. In nitrogen two decomposition steps were observed: the loss of crystallization water, respectively the decomposition of the phenyl-vinyl radical. In air, the same dehydration was observed as the first step, but the second one is a thermooxidation of the organic radical with formation of the pyrophosphoric anion.
The kinetic analysis of the TG non-isothermal data was performed by the isoconversional methods suggested by Friedman and Flynn, Wall and Ozawa, as well as by the non-parametric (Sempere-Nomen) method. All processes put in evidence in TG curves exhibit strong changes of the activation energy values with the conversion degree, which mean that these processes are complex ones. Assuming that each of these processes consists in two steps, the application of non-parametric method leads to average values of the activation energy close to the average values of this parameter obtained by isoconversional methods.
Similar content being viewed by others
References
A. Clearfield, Progres in Inorganic Chemistry, K. D. Karbin, Ed., John Wiley and Sons, NY 1998, Vol. 47, p. 371.
B. Bujoli, P. Palvadeau and J. Rouxel, Chem. Mater., 2 (1990) 582.
D. M. Poojary, Y. P. Zhang, B. Zhang and A. Clearfield, Chem. Mater., 7 (1995) 822.
D. Grohol, M. A. Subramanian, M. A. Poojary and A. Clearfield, Inorg. Chem., 35 (1996) 5264.
G. Cao, H. Lee, V. M. Lynch, I. S. Swinnea and T. E. Mallouk, Inorg. Chem., 27 (1988) 2781.
G. Cao, H. Lee, V. M. Lynch, I. S. Swinnea and T. E. Mallouk, Inorg. Chem., 29 (1990) 2112.
A. Cabeza, M. A. G. Aranda, S. Bruque, M. D. Poojary, A. Clearfield and I. Sanz, Inorg. Chem., 37 (1998) 4168.
I. Le. Bideau, C. Payen, P. Palvadeau and B. Buyoli, Inorg. Chem., 33 (1994) 4885.
S. Drumel, P. Jonvier, D. Deniaud and B. Buyoli, I. Chem. Soc., Chem. Commun., (1995) 1051.
R. M. Silverstein, G. Clayton Basster and T. Morrell, ’Spectrometric Identification of the Compounds’, 5th Ed., John Wiley, 1995.
I. H. Flynn and L. A. Wall, Polym. Lett., 4 (1966) 323.
T. Ozawa, Bull. Chem. Soc. Jpn., 38 (1965) 1881.
H. L. Friedman, J. Polym. Sci., 6C (1965) 183.
R. Serra, R. Nomen and J. Sempere, J. Therm. Anal. Cal., 52 (1998) 933.
R. Serra, J. Sempere and R. Nomen, Thermochim. Acta, 316 (1998) 37.
J. Sempere, R. Nomen and R. Serra, J. Therm. Anal. Cal., 56 (1999) 843.
P. Budrugeac, D. Homentcovschi and E. Segal, J. Therm. Anal. Cal., 66 (2001) 557.
P. Budrugeac and E. Segal, Int. J. Chem. Kinet., 33 (2001) 564.
T. Vlase, G. Vlase, N. Doca and C. Bolcu, J. Therm. Anal. Cal., 80 (2005) 59.
T. Vlase, G. Vlase, M. Doca and N. Doca, J. Therm. Anal. Cal., 80 (2005) 207.
T. Vlase, G. Vlase and N. Doca, J. Therm. Anal. Cal., 80 (2005) 425.
T. Vlase, G. Vlase, N. Birta and N. Doca, J. Therm. Anal. Cal., 88 (2007) 631.
M. E. Wall, Singular value decomposition and principal component analysis, A practical approach to microarray data analysis, 9. 91-109, Kluwer-Norwel, MA (2003). LANL LA-UR-02.
J. Šesták and G. Berggren, Thermochim. Acta, 3 (1971).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Doca, N., Vlase, G., Vlase, T. et al. Thermal behavior of Cd2+ and Co2+ phenyl-vinyl-phosphonates under non-isothermal condition. J Therm Anal Calorim 94, 441–445 (2008). https://doi.org/10.1007/s10973-008-9347-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-008-9347-x