Summary
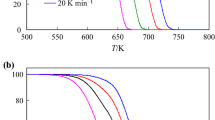
The thermal behavior of KH2PO4, NaH2PO4 and Na2HPO4 under non-isothermal conditions using TG method with different heating rates was studied. The values of the reaction rate were processed by means of Friedman’s differential-isoconversional method. A dependence of the activation energy vs. conversion was observed. Therefore a procedure based on the compensation effect (suggested by Budrugeac and Segal) was applied. A less speculative data processing protocol was offered by the non-parametric kinetics method suggested by Serra, Nomen and Sempere. Three steps were observed by non-isothermal heating: a dehydration, a dimerization and a polycondensation. The differences in the intimate reaction mechanism are determined by the initial number of water molecules.
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Vlase, T., Vlase, G. & Doca, N. Kinetics of thermal decomposition of alkaline phosphates. J Therm Anal Calorim 80, 207–210 (2005). https://doi.org/10.1007/s10973-005-0637-2
Issue Date:
DOI: https://doi.org/10.1007/s10973-005-0637-2