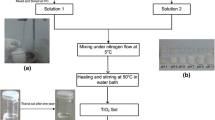
Abstract
Titania is a versatile semiconductor widely used in many applications, including photocatalysis. Among the processing routes used to prepare titania, the sol–gel process deserves to be highlighted because it allows obtaining samples with tailored composition, particle size, and specific surface area. Such properties play a key role in the photocatalytic behavior of titania. In this study, we show that by adjusting the sol–gel parameters and drying/heat treatment conditions it is possible to control the co-existence of anatase and rutile in the synthesized sol–gel titania. The works found in the literature usually deal with the preparation of crystalline titania using water-rich solutions, which usually gives rise to anatase. However, we demonstrate that the lack of water in the starting solution favors the formation of both anatase and rutile; the co-existence of these two phases has been reported to improve the photocatalytic behavior of titania due to a band-gap alignment. Furthermore, we propose a sol–gel route based on a single-step hydrothermal procedure where nanoparticles with sizes of about 3 nm and surface areas up to 120 m2.g−1 are obtained. This study accounts for the knowledge regarding the preparation and use of sol–gel titania for photocatalysis applications. It is supported by a series of experimental techniques, including X-ray diffraction, N2 sorption, thermogravimetry, differential thermal analysis, Raman spectroscopy, transmission electron microscopy, selected area electron diffraction, and UV–Vis diffuse reflectance spectroscopy. The photoactivity of the prepared samples was evaluated in terms of the photodegradation of methylene blue under near-ultraviolet (UV-A) light.

Graphical abstract
Highlights
-
Titania nanoparticles obtained from a sol–gel route based on a water-deficient starting solution.
-
Crystalline samples prepared by either heat-treatment or hydrothermal treatment.
-
The lack of water allows the preparation of anatase-rutile mixed titania.
-
Nanoparticles with sizes as small as 3 nm and specific surface areas up to 120 m2.g−1.
-
Photoactivty evaluated in terms of the degradation of methylene blue under UV-A light.







Similar content being viewed by others
References
Gonçalves BS, Palhares HG, de Souza TCC et al. (2019) Effect of the carbon loading on the structural and photocatalytic properties of reduced graphene oxide-TiO2 nanocomposites prepared by hydrothermal synthesis. J Mater Res Technol 8:6262–6274. https://doi.org/10.1016/j.jmrt.2019.10.020
Gonçalves BS, Souza TCC, de, Castro VGde et al. (2019) Solvent effect on the structure and photocatalytic behavior of TiO2-RGO nanocomposites. J Mater Res 34:3918–3930. https://doi.org/10.1557/jmr.2019.342
Silva LLO, Vasconcelos DCL, Nunes EHM et al. (2012) Processing, structural characterization and performance of alumina supports used in ceramic membranes. Ceram Int 38:1943–1949. https://doi.org/10.1016/j.ceramint.2011.10.025
Kim DY, Joshi BN, Park JJ et al. (2014) Graphene-titania films by supersonic kinetic spraying for enhanced performance of dye-sensitized solar cells. Ceram Int 40:11089–11097. https://doi.org/10.1016/j.ceramint.2014.03.131
Houmard M, Vasconcelos DCL, Vasconcelos WL et al. (2009) Water and oil wettability of hybrid organic-inorganic titanate-silicate thin films deposited via a sol-gel route. Surf Sci 603:2698–2707. https://doi.org/10.1016/j.susc.2009.07.005
Mahy JG, Léonard GL-M, Pirard S et al. (2017) Aqueous sol–gel synthesis and film deposition methods for the large-scale manufacture of coated steel with self-cleaning properties. J Sol Gel Sci Technol 81:27–35. https://doi.org/10.1007/s10971-016-4020-5
Bu S, Cui C, Liu X, Bai L (2007) Preparation of nanocrystalline porous titania films on titanium substrates by a sol–gel method with polyethylene glycol as a template. J Sol Gel Sci Technol 43:151–159. https://doi.org/10.1007/s10971-007-1556-4
Farrokhi-Rad M (2018) Electrophoretic deposition of titania nanostructured coatings with different porous patterns. Ceram Int 44:15346–15355. https://doi.org/10.1016/j.ceramint.2018.05.184
Xiang C, Jiang D, Zou Y et al. (2015) Ammonia sensor based on polypyrrole-graphene nanocomposite decorated with titania nanoparticles. Ceram Int 41:6432–6438. https://doi.org/10.1016/j.ceramint.2015.01.081
Jing Z, Ling B, Yu Y et al. (2015) Preparation and gas sensing activity of La and Y co-doped titania nanoparticles. J Sol Gel Sci Technol 73:112–117. https://doi.org/10.1007/s10971-014-3501-7
Hanaor DAH, Sorrell CC (2011) Review of the anatase to rutile phase transformation. J Mater Sci 46:855–874
Liu L, Zhao H, Andino JM, Li Y (2012) Photocatalytic CO2 reduction with H2O on TiO2 nanocrystals: comparison of anatase, rutile, and brookite polymorphs and exploration of surface chemistry. ACS Catal 2:1817–1828. https://doi.org/10.1021/cs300273q
Siah WR, Lintang HO, Shamsuddin M, Yuliati L (2016) High photocatalytic activity of mixed anatase-rutile phases on commercial TiO2 nanoparticles. IOP Conf Ser Mater Sci Eng 107:12005. https://doi.org/10.1088/1757-899x/107/1/012005
He J, Du Y, Bai Y et al. (2019) Facile formation of anatase/rutile TiO2 nanocomposites with enhanced photocatalytic activity. Molecules 24:2996. https://doi.org/10.3390/molecules24162996
Ishigaki T, Nakada Y, Tarutani N et al. (2020) Enhanced visible-light photocatalytic activity of anatase-rutile mixed-phase nano-size powder given by high-temperature heat treatment. R Soc Open Sci 7:191539. https://doi.org/10.1098/rsos.191539
Konaka R, Kasahara E, Dunlap WC et al. (1999) Irradiation of titanium dioxide generates both singlet oxygen and superoxide anion. Free Radic Biol Med 27:294–300. https://doi.org/10.1016/S0891-5849(99)00050-7
Zhang J, Zhou P, Liu J, Yu J (2014) New understanding of the difference of photocatalytic activity among anatase, rutile and brookite TiO2. Phys Chem Chem Phys 16:20382–20386. https://doi.org/10.1039/c4cp02201g
Miao G, Chen L, Qi Z (2012) Facile synthesis and active photocatalysis of mesoporous and microporous TiO2 nanoparticles. Eur J Inorg Chem 5864–5871. https://doi.org/10.1002/ejic.201200833
Ullattil SG, Periyat P (2017) Sol-gel synthesis of titanium dioxide. In: Pillai SC, Hehir S (eds) Sol-gel materials for energy, environment and electronic applications. Springer International Publishing, Cham, pp 271–283
Nagliati M, Carotta MC, Gherardi S et al. (2006) TiO2 nanopowders for sensing applications; A comparison between traditional and hydrothermal synthesis way. Adv Sci Technol 45:205–208. https://doi.org/10.4028/www.scientific.net/ast.45.205
Seck EI, Doña-Rodríguez JM, Pulido Melián E et al. (2013) Comparative study of nanocrystalline titanium dioxide obtained through sol-gel and sol-gel-hydrothermal synthesis. J Colloid Interface Sci 400:31–40. https://doi.org/10.1016/j.jcis.2013.03.019
Wong CL, Tan YN, Mohamed AR (2011) A review on the formation of titania nanotube photocatalysts by hydrothermal treatment. J Environ Manag 92:1669–1680. https://doi.org/10.1016/j.jenvman.2011.03.006
López R, Gómez R (2012) Band-gap energy estimation from diffuse reflectance measurements on sol-gel and commercial TiO2: a comparative study. J Sol Gel Sci Technol 61:1–7. https://doi.org/10.1007/s10971-011-2582-9
Hargreaves JSJ (2016) Some considerations related to the use of the Scherrer equation in powder X-ray diffraction as applied to heterogeneous catalysts. Catal Struct React 2:33–37. https://doi.org/10.1080/2055074X.2016.1252548
Bregadiolli BA, Fernandes SL, Graeff CF, de O (2017) Easy and fast preparation of TiO2-based nanostructures using microwave assisted hydrothermal synthesis. Mater Res 20:912–919. https://doi.org/10.1590/1980-5373-MR-2016-0684
Catauro M, Tranquillo E, Dal Poggetto G, et al. (2018) Influence of the heat treatment on the particles size and on the crystalline phase of TiO2 synthesized by the sol-gel method. Materials 11:. https://doi.org/10.3390/ma11122364
Tang H, Prasad K, Sanjinès R et al. (1994) Electrical and optical properties of TiO2 anatase thin films. J Appl Phys 75:2042–2047. https://doi.org/10.1063/1.356306
Song P, Zhang XY, Sun MX et al. (2012) Graphene oxide modified TiO2 nanotube arrays: enhanced visible light photoelectrochemical properties. Nanoscale 4:1800–1804. https://doi.org/10.1039/c2nr11938b
Tamilselvan V, Yuvaraj D, Rakesh Kumar R, Narasimha Rao K (2012) Growth of rutile TiO2 nanorods on TiO2 seed layer deposited by electron beam evaporation. Appl Surf Sci 258:4283–4287. https://doi.org/10.1016/j.apsusc.2011.12.079
Tuschel D (2019) Raman spectroscopy and polymorphism. Spectroscopy 34:10–21
Challagulla S, Tarafder K, Ganesan R, Roy S (2017) Structure sensitive photocatalytic reduction of nitroarenes over TiO2. Sci Rep 7:8783. https://doi.org/10.1038/s41598-017-08599-2
Silva LMC, Gonçalves BS, Braga JDO et al. (2021) Preparation of titania-reduced graphene oxide composite coatings with electro- and photosensitive properties. Appl Surf Sci 538:148029. https://doi.org/10.1016/j.apsusc.2020.148029
Shaikh SF, Mane RS, Min BK et al. (2016) D-sorbitol-induced phase control of TiO2 nanoparticles and its application for dye-sensitized solar cells. Sci Rep 6:20103. https://doi.org/10.1038/srep20103
Topcu S, Jodhani G, Gouma P (2016) Optimized nanostructured TiO2 photocatalysts. Front Mater 3:35. https://doi.org/10.3389/fmats.2016.00035
So WW, Park SBin, Kim KJ et al. (2001) The crystalline phase stability of titania particles prepared at room temperature by the sol-gel method. J Mater Sci 36:4299–4305. https://doi.org/10.1023/A:1017955408308
Kumar KNP, Keizer K, Burggraaf AJ (1993) Textural evolution and phase transformation in titania membranes: Part 1.—unsupported membranes. J Mater Chem 3:1141–1149. https://doi.org/10.1039/JM9930301141
Lopez-Iscoa P, Pugliese D, Boetti NG et al. (2018) Design, synthesis, and structure-property relationships of Er3+-doped TiO2 luminescent particles synthesized by sol-gel. Nanomaterials 8:20. https://doi.org/10.3390/nano8010020
Ying L, Hon LS, White T et al. (2003) Controlled nanophase development in photocatalytic titania. Mater Trans 44:1328–1332. https://doi.org/10.2320/matertrans.44.1328
Thommes M, Kaneko K, Neimark AV et al. (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl Chem 87:1051–1069. https://doi.org/10.1515/pac-2014-1117
Konstantinou IK, Albanis TA (2004) TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations: a review. Appl Catal B Environ 49:1–14. https://doi.org/10.1016/j.apcatb.2003.11.010
Soares WM, Athayde DD, Nunes EHM (2018) LCA study of photovoltaic systems based on different technologies. Int J Green Energy 15:577–583. https://doi.org/10.1080/15435075.2018.1510408
Saqib N us, Adnan R, Shah I (2016) A mini-review on rare earth metal-doped TiO2 for photocatalytic remediation of wastewater. Environ Sci Pollut Res 23:15941–15951. https://doi.org/10.1007/s11356-016-6984-7
Li C, Zhao Z, Shindume Lomboleni H et al. (2017) Enhanced visible photocatalytic activity of nitrogen doped single-crystal-like TiO2 by synergistic treatment with urea and mixed nitrates. J Mater Res 32:737–747. https://doi.org/10.1557/jmr.2016.448
Lyu Z, Liu B, Wang R, Tian L (2017) Synergy of palladium species and hydrogenation for enhanced photocatalytic activity of {001} facets dominant TiO2 nanosheets. J Mater Res 32:2781–2789. https://doi.org/10.1557/jmr.2017.232
Scanlon DO, Dunnill CW, Buckeridge J et al. (2013) Band alignment of rutile and anatase TiO2. Nat Mater 12:798–801. https://doi.org/10.1038/nmat3697
Yanagisawa K, Ovenstone J (1999) Crystallization of anatase from amorphous titania using the hydrothermal technique: effects of starting material and temperature. J Phys Chem B 103:7781–7787. https://doi.org/10.1021/jp990521c
Bubacz K, Choina J, Dolat D, Morawski AW (2010) Methylene blue and phenol photocatalytic degradation on nanoparticles of anatase TiO2. Pol J Environ Stud 19:685–691
Tichapondwa SM, Newman JP, Kubheka O (2020) Effect of TiO2 phase on the photocatalytic degradation of methylene blue dye. Phys Chem Earth, Parts A/B/C 118–119:102900. https://doi.org/10.1016/j.pce.2020.102900
Boehme M, Ensinger W (2011) Mixed phase anatase/rutile titanium dioxide nanotubes for enhanced photocatalytic degradation of methylene-blue. Nano-Micro Lett 3:236–241. https://doi.org/10.1007/BF03353678
Arbuj SS, Hawaldar RR, Mulik UP et al. (2010) Preparation, characterization and photocatalytic activity of TiO2 towards methylene blue degradation. Mater Sci Eng B 168:90–94. https://doi.org/10.1016/j.mseb.2009.11.010
Acknowledgements
EHMN is grateful for the financial support from CAPES (PROEX), FAPEMIG (RED-00102-16, RENOVAMIN), and CNPq (304415/2021-9). The authors thank the technical support from Taiane Guedes/INCT Acqua (Raman spectroscopy) and the UFMG microscopy center (TEM). CeNano2I/CEMUCASI (Prof. Herman Mansur and Dr. Alexandra Mansur) are also recognized for their technical support to this research (TG/DTA).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Marra, M., Dumont, M., Palhares, H.G. et al. Structural and photocatalytic properties of sol–gel-derived TiO2 samples prepared by conventional and hydrothermal methods using a low amount of water. J Sol-Gel Sci Technol 103, 97–107 (2022). https://doi.org/10.1007/s10971-022-05780-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-022-05780-6