Abstract
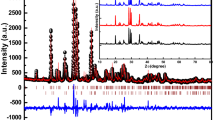
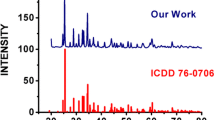
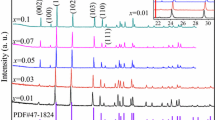
Phase pure monoclinic CaAl2O4 (CA2), CaAl4O7 (CA4) and hexagonal CaAl12O19 (CA12) doped with 1 mol% Ce3+ phosphor were prepared by combustion method at 500 °C in a few minutes. Synthesized phosphor has been well characterized by X-ray diffraction. In the present paper, we discussed the comparative luminescence properties of the phosphors with respect to their crystal structures and the various sites of Ca2+. This paper provides evidence for different coordinated sites of Ca2+ ion in the host used. Comparison between calculated and experimental value of position of energy in the lower d-band edge for Ce3+ ion is discussed. Ce3+ ions were doped in order to study the emission characteristics in the prepared sample with respect to their crystal symmetry. Different emission spectrum is observed despite of their same crystal structure. The photoluminescence emission spectra of the CaAl2O4:Ce, CaAl4O7:Ce, and CaAl12O19:Ce phosphors show strong Ce3+ emission at around 370, 330, and 320 nm for the excitation at 300, 273, and 264 nm wavelengths respectively. The emission characteristics are credited to 5d→4f (2F5/2 and 2F7/2) type transitions in Ce3+.
Graphical Abstract











Similar content being viewed by others
References
Busta HH (2001) Field emission flat panel displays. Vac Microelectron 5:289–343
Nagabhushana H, Sharma SC, Prashantha SC, Sunitha DV et al. (2014) CdSiO3:Eu3+ red nanophosphors prepared by low temperature solution combustion technique, its structural and luminescent properties. J Alloys Compd 616:284–292
Kumar A, Dhoble SJ, Peshwe DR, Bhatt J (2014) Structural and photoluminescence properties of nepheline-structure NaAlSiO4: Dy3+ nanophosphors. J Alloys Compd 609:100–106
Yadav DS (2012) Electronic and mechanical properties of rare earth monochalcogenides. J Alloys Compd 537:250–254
Yao G, Su L, Xu X, Xu J (2008) Eu:Y2O3 nano-phosphor prepared by novel energy-saving solution combustion method. J Alloys Compd 462:381–385
Trojan-Piegza J, Zych E (2004) Preparation of nanocrystalline Lu2O3:Eu phosphor via a molten salts route. J Alloys Compd 380:118–122
Uematsu K, Ochiai A, Toda K, Sato M (2006) Characterization of YVO4:Eu3+ phosphors synthesized by microwave heating method. J Alloys Compd 408:860–863
Nissamudeen KM, Gopchandran KG (2010) Y2O3:Eu3+-based nanophosphors with higher oscillator strength through lithium incorporation and indirect oxidation. J Alloys Compd 490:399–406
Jia D, Wu B, Zhu J (2000) Correction of excitation spectra of long persistent phosphors. J Lumin 90:33–37
Jia D, Zhu J, Wu B (2000) Improvement of persistent phosphorescence of Ca0.9Sr0.1S:Bi3+ by codoping Tm3+. J Lumin 91:59–65
Halefoolu YZ, Kusvuran E (2010) J Ceram Proc Res 11:92
Patil KC, Aruna ST, Mimani T (2002) Combustion synthesis: an update. Solid State Mater Sci 6:507–512
Horkner W, Muller HK (1976) Zur kristallstruktur von CaAl2O4. J Inorg Nucl Chem 38:983–984
Goodwin DW, Lindop AJ (1970) The crystal structure of CaO2Al2O3. Acta Cryst B 26:1230–1235
Utsunomiya A, Tanaka K, Morikawa H et al. (1988) Structure refinement of CaO·6Al2O3. J Solid State Chem 75:197–200
Brik MG, Pan YX, Liu GK (2011) Spectroscopic and crystal field analysis of absorption and photoluminescence properties of red phosphor CaAl12O19:Mn4+ modified by MgO. J Alloys Compd 509:1452–1456
Singh V, Rai VK, Lee IJ, Ledoux-Rak I, Al-Shamery K, Nordmann J, Haase M (2012) Infrared, visible and upconversion emission of CaAl12O19 powders doped with Er3+, Yb3+, and Mg2+ ions. Appl Phys B 106:223–228
Justel T, Nikol H, Ronda C, Angew (1998) New developments in the field of luminescent materials for lighting and displays. Chem Int Ed 37:3084
Hoffman MV (1971) Effect of thorium on Ce+3 phosphors. J Electrochem Soc 118:1508–1510
Jia D, Meltzer RS, Yen WM, Jia W, Wang X (2002) Green phosphorescence of CaAl2O4: Tb3+,Ce3+ through persistence energy transfer. Appl Phys Lett 80:1535–1537
Kingsley JJ, Manickam N, Patil KC (1990) Combustion synthesis and properties of fine particle fluorescent aluminous oxides. Bull Mater Sci 13:179–189
Zhang K, Hu W, Wu Y, Liu H (2008) Phys Rev B 404:1678
van Pieterson L, Reid MF, Wegh RT, Soverna S, Meijerink A (2002) 4fn→4fn−15d transitions of the light lanthanides. Phys Rev B 65:045113
van Pieterson L, Reid MF, Burdick GW, Meijerink A (2002) 4fn→4fn−15d transitions of the heavy lanthanides. Phys Rev B 65:045114
Buerger MJ (1954) The stuffed derivatives of the silica stuctures. Am Miner 39:600
Ravichandran D, Johnson ST, Erdei S, Roy R et al. (1999) Crystal chemistry and luminescence of the Eu2+-activated alkaline earth aluminate phosphors. Displays 19:197–203
Kato K, Saalfeld H (1968) Verfeinerung der Kristallstrucktur von CaO·6Al2O3. Neues Jahr Miner Abh 109:192–299
Kuklja MM (2000) Defects in yttrium aluminium perovskite and garnet crystals: atomistic study. J Phys Condens Matter 12:2953
Patel AP, Levy MR, Grimes RW, Gaume RM, Frigelson RS, McClellan KJ, Stanek CR (2008) Mechanisms of nonstoichiometry in Y3Al5O12. Appl Phys Lett 93:191902
Dong J, Lu K (1991) Noncubic symmetry in garnet structures studied using extended X-ray-absorption fine-structure spectra. Phys Rev B 43:8808
Truong D, Devaraju MK, Tomai T, Honma I (2013) Direct observation of in LiCoPO4 cathode materials by annular dark. ACS Appl Mater Interfaces 5:9926–9932
Basavaraju N, Priolkar KR, Gourier D, Sharma SK, Bessiere A, Viana B (2015) The importance of inversion disorder in the visible light induced persistent luminescence in Cr3+ doped AB2O4 (A=Zn or Mg and B=Ga or Al). Phys Chem Chem Phys 17:1790–1799
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst A 32:751–767
Van Uitert LG (1984) An empirical relation fitting the position in energy of the lower d-band edge for Eu2+ OR Ce3+ in various compounds. J Lumin 29:1–9
Lin Y, Zhang Z, Tang Z, Zhang J, Zheng Z, Lu X (2001) The characterization and mechanism of long afterglow in alkaline earth aluminates phosphors co-doped by Eu2O3 and Dy2O3. Mater Chem Phys 70:156–159
Acknowledgments
We are thankful to Board of Research in Nuclear Sciences (BRNS), Department of Atomic Energy, Govt. of India, for providing financial assistance under the grant 2013/36/09-BRNS dated 30/05/2013.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Gedekar, K.A., Wankhede, S.P. & Moharil, S.V. Synthesis and comparative study of Ce3+ ion in calcium aluminates. J Sol-Gel Sci Technol 82, 344–351 (2017). https://doi.org/10.1007/s10971-017-4341-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-017-4341-z