Abstract
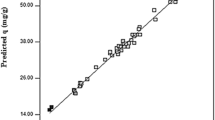
The strontium (II) uptake on Chlorella vulgaris was studied in a batch system. The interactive effects of parameters such as pretreatment, initial pH, temperature, initial concentration, and biosorbent dosage, on the biosorption of strontium were analyzed using the response surface methodology. The biosorption capacity of the algae at optimal conditions of initial pH 7.4, biosorbent dosage 1 g/L, and initial strontium concentration 300 mg/L for Ca-treated biomass was found to be 98.33 mg/g. Investigation of the kinetics showed that the biosorption process follows the pseudo-second-order model. Also the equilibrium of the process showed a better fit by the Langmuir isotherm.








Similar content being viewed by others
References
Apted MJ, Ahn J (2017) Repository 101: multiple-barrier geological repository design and isolation strategies for safe disposal of radioactive materials. Geological repository systems for safe disposal of spent nuclear fuels and radioactive waste. Elsevier, pp 3–26
Liang L, Guixiang Y (2021) Study on aging management of operating nuclear power plants in China. In: International conference on nuclear engineering. American Society of Mechanical Engineers, p V002T006A003
Pathak P, Gupta DK (2020) Strontium contamination in the environment. Springer
Singh N, Nagpal G, Agrawal S (2018) Water purification by using adsorbents: a review. Environ Technol Innov 11:187–240
Feng J, Zhao X, Zhou H, Qiu L, Dai Y, Luo H, Otero M (2020) Removal of strontium by high-performance adsorbents Saccharomyces cerevisiae-Fe3O4 bio-microcomposites. J Radioanal Nucl Chem 326:525–535
Garbowski T, Charazińska S, Pulikowski K, Wiercik P (2020) Application of microalgae cultivated on pine bark for the treatment of municipal wastewater in cylindrical photobioreactors. Water Environ J 34:949–959
Ji Y-Q, Hu Y-T, Tian Q, Shao X-Z, Li J, Safarikova M, Safarik I (2010) Biosorption of strontium ions by magnetically modified yeast cells. Sep Sci Technol 45:1499–1504
Medfu Tarekegn M, Zewdu Salilih F, Ishetu AI (2020) Microbes used as a tool for bioremediation of heavy metal from the environment. Cogent Food Agric 6:1783174
Priya A, Gnanasekaran L, Dutta K, Rajendran S, Balakrishnan D, Soto-Moscoso M (2022) Biosorption of heavy metals by microorganisms: evaluation of different underlying mechanisms. Chemosphere 307:135957
Rai J, Kumar D, Gaur J (2019) Sorption of malachite green (a cationic dye) and heavy metals by dead biomass of Phormidesmis molle (cyanobacteria)-dominated mat. Water Environ J 33:51–60
Zheng X, Hu P, Yao R, Cheng J, Chang Y, Mei H, Sun S, Chen S, Wen H (2022) Biosorption behavior and biomineralization mechanism of low concentration uranium (VI) by pseudomonas fluorescens. J Radioanal Nucl Chem 331:4675–4684
Wang Z, Chen C, Liu H, Hrynshpan D, Savitskaya T, Chen J, Chen J (2020) Enhanced denitrification performance of Alcaligenes sp. TB by Pd stimulating to produce membrane adaptation mechanism coupled with nanoscale zero-valent iron. Sci Total Environ 708:135063
Banerjee S, Kundu A, Dhak P (2022) Bioremediation of uranium from waste effluents using novel biosorbents: a review. J Radioanal Nucl Chem 331:2409–2435
Jacob JM, Karthik C, Saratale RG, Kumar SS, Prabakar D, Kadirvelu K, Pugazhendhi A (2018) Biological approaches to tackle heavy metal pollution: a survey of literature. J Environ Manag 217:56–70
Khani M (2013) Dynamics and thermodynamics studies on the lead and cadmium removal from aqueous solutions by Padina sp. algae: studies in single and binary metal systems. Sep Sci Technol 48:2688–2699
Khani M, Pahlavanzadeh H, Alizadeh K (2012) Biosorption of strontium from aqueous solution by fungus Aspergillus terreus. Environ Sci Pollut Res 19:2408–2418
Singh A, Pal DB, Mohammad A, Alhazmi A, Haque S, Yoon T, Srivastava N, Gupta VK (2022) Biological remediation technologies for dyes and heavy metals in wastewater treatment: new insight. Biores Technol 343:126154
Amenorfenyo DK, Huang X, Li C, Li F, Zeng Q, Zhang N, Xie L, Wang P (2020) A review of microalgae and other treatment methods of distillery wastewater. Water Environ J 34:988–1002
Hassan Khani M, Reza Keshtkar A, Meysami B, Firouz Zarea M, Jalali R (2006) Biosorption of uranium from aqueous solutions by nonliving biomass of marinealgae Cystoseira indica. Electron J Biotechnol 9:100. https://doi.org/10.4067/S0717-34582006000200003
Khani M (2012) Biosorption of strontium by a nonliving brown marine agae, Padina sp. Sep Sci Technol 47:1886–1897
Khani M (2013) Biosorption of strontium by Padina sp. algae biomass: process optimisation and equilibrium study. Int J Environ Technol Manag 16:290–311
Khani M, Keshtkar A, Ghannadi M, Pahlavanzadeh H (2008) Equilibrium, kinetic and thermodynamic study of the biosorption of uranium onto Cystoseria indica algae. J Hazard Mater 150:612–618
Khani MH (2011) Uranium biosorption by Padina sp. algae biomass: kinetics and thermodynamics. Environ Sci Pollut Res 18:1593–1605
Khani MH (2011) Statistical analysis and isotherm study of uranium biosorption by Padina sp. algae biomass. Environ Sci Pollut Res 18:790–799
Orabi AH, Abdelhamid AE-S, Salem HM, Ismaiel DA (2020) New adsorptive composite membrane from recycled acrylic fibers and Sargassum dentifolium marine algae for uranium and thorium removal from liquid waste solution. J Radioanal Nucl Chem 326:1233–1247
Padri M, Boontian N, Piasai C (2022) Comparison of single strain, natural algal communities and a native algal bloom: application for wastewater treatment and biomass generation in cassava biogas effluent. Water Environ J 36:679–693
Prabhu AA, Chityala S, Jayachandran D, Deshavath NN, Veeranki VD (2021) A two step optimization approach for maximizing biosorption of hexavalent chromium ions (Cr (VI)) using alginate immobilized Sargassum sp in a packed bed column. Sep Sci Technol 56:90–106
Jampílek J, Kráľová K (2021) Seaweeds as indicators and potential remediators of metal pollution. Plant growth-promoting microbes for xustainable biotic and abiotic stress management. Springer, pp 51–92
Park S, Lee M (2017) Removal of copper and cadmium in acid mine drainage using Ca-alginate beads as biosorbent. Geosci J 21:373–383
Khamseh AAG, Ghorbanian SA, Amini Y, Shadman MM (2023) Investigation of kinetic, isotherm and adsorption efficacy of thorium by orange peel immobilized on calcium alginate. Sci Rep 13:8393. https://doi.org/10.1038/s41598-023-35629-z
Khamseh AG, Ghorbanian SA (2018) Experimental and modeling investigation of thorium biosorption by orange peel in a continuous fixed-bed column. J Radioanal Nucl Chem 317:871–879
Zhu W, Li Y, Yu Y, Duan T, Zhou D, Wang L, Zhou J, Kuang M (2018) Environment-friendly bio-materials based on cotton-carbon aerogel for strontium removal from aqueous solution. J Radioanal Nucl Chem 316:553–560
Wang Z, Hu L, Zhao M, Dai L, Hrynsphan D, Tatsiana S, Chen J (2022) Bamboo charcoal fused with polyurethane foam for efficiently removing organic solvents from wastewater: experimental and simulation. Biochar 4:28
Javanbakht V, Alavi SA, Zilouei H (2014) Mechanisms of heavy metal removal using microorganisms as biosorbent. Water Sci Technol 69:1775–1787
Behera SK, Meena H, Chakraborty S, Meikap B (2018) Application of response surface methodology (RSM) for optimization of leaching parameters for ash reduction from low-grade coal. Int J Min Sci Technol 28:621–629
Ghelich R, Jahannama MR, Abdizadeh H, Torknik FS, Vaezi MR (2019) Central composite design (CCD)-Response surface methodology (RSM) of effective electrospinning parameters on PVP-B-Hf hybrid nanofibrous composites for synthesis of HfB2-based composite nanofibers. Compos B Eng 166:527–541
Hafeez A, Taqvi SAA, Fazal T, Javed F, Khan Z, Amjad US, Bokhari A, Shehzad N, Rashid N, Rehman S (2020) Optimization on cleaner intensification of ozone production using Artificial Neural Network and Response Surface Methodology: parametric and comparative study. J Clean Prod 252:119833
Khamseh AAG, Amini Y, Shademan MM, Ghazanfari V (2023) Intensification of thorium biosorption onto protonated orange peel using the response surface methodology. Chem Prod Process Model 10:200. https://doi.org/10.1515/cppm-2022-0085
Ugwu EI, Agunwamba JC (2022) Optimization of process parameters for adsorption of hexavalent chromium from wastewater using response surface methodology. Int J Eng Res Afr 59:239–262
Yarahmadi A, Khani MH, Nasiri Zarandi M, Amini Y (2023) Ce (ΙΙΙ) and La (ΙΙΙ) ions adsorption through Amberlite XAD-7 resin impregnated via CYANEX-272 extractant. Sci Rep 13:6930
Soleymani F, Khani M, Pahlevanzadeh H, Amini Y (2023) Intensification of strontium (II) ion biosorption on Sargassum sp via response surface methodology. Sci Rep 13:5403
Zarouk C (1966) Contribution a l’etude d’une cyanophycee. Influence de Divers Facteurs Physiques et Chimiques sur las Croissance et la Photosynthese de Spirulina maxima. University of Paris, France
Mahmoud A, Fawzy M, Hosny G, Obaid A (2021) Equilibrium, kinetic, and diffusion models of chromium (VI) removal using Phragmites australis and Ziziphus spina-christi biomass. Int J Environ Sci Technol 18:2125–2136
Hashemipour N, Karimi-Sabet J, Motahari K, Monfared SM, Amini Y, Moosavian MA (2018) Experimental and simulation investigation on separation of binary hydrocarbon mixture by thermogravitational column. J Mol Liq 268:791–806
Khalifa EB, Rzig B, Chakroun R, Nouagui H, Hamrouni B (2019) Application of response surface methodology for chromium removal by adsorption on low-cost biosorbent. Chemom Intell Lab Syst 189:18–26
Marsousi S, Karimi-Sabet J, Moosavian MA, Amini Y (2019) Liquid-liquid extraction of calcium using ionic liquids in spiral microfluidics. Chem Eng J 356:492–505
Jahromi PF, Karimi-Sabet J, Amini Y (2018) Ion-pair extraction-reaction of calcium using Y-shaped microfluidic junctions: an optimized separation approach. Chem Eng J 334:2603–2615
Moazzen N, Khanmohammadi M, Bagheri Garmarudi A, Kazemipour M, Ansari Dogaheh M (2019) Optimization and infrared spectrometric evaluation of the mechanical properties of PLA-based biocomposites. J Macromol Sci Part A 56:17–25
Dvoretsky D, Akulinin E, Dvoretsky S, Temnov M, Androsova A (2016) Defining optimal conditions for Chlorella vulgaris microalgae biomass cell walls disruption in the process of biofuel production. In: Proceedings of the 16th international multidisciplinry scientific geoconference SGEM, pp 261–267
Wayne RO (2009) Plant cell biology: from astronomy to zoology. Academic Press
Tattibayeva Z, Tazhibayeva S, Kujawski W, Zayadan B, Musabekov K (2022) Peculiarities of adsorption of Cr (VI) ions on the surface of Chlorella vulgaris ZBS1 algae cells. Heliyon 8:e10468
Bardestani R, Roy C, Kaliaguine S (2019) The effect of biochar mild air oxidation on the optimization of lead (II) adsorption from wastewater. J Environ Manag 240:404–420
Es-Sahbany H, Hsissou R, El Hachimi M, Allaoui M, Nkhili S, Elyoubi M (2021) Investigation of the adsorption of heavy metals (Cu Co, Ni and Pb) in treatment synthetic wastewater using natural clay as a potential adsorbent (Sale-Morocco). Mater Today Proc 45:7290–7298
Sahu UK, Mahapatra SS, Patel RK (2018) Application of Box-Behnken Design in response surface methodology for adsorptive removal of arsenic from aqueous solution using CeO2/Fe2O3/graphene nanocomposite. Mater Chem Phys 207:233–242
Özer A, Gürbüz G, Çalimli A, Körbahti BK (2009) Biosorption of copper (II) ions on Enteromorpha prolifera: application of response surface methodology (RSM). Chem Eng J 146:377–387
Özer A, Gürbüz G, Çalimli A, Körbahti BK (2008) Investigation of nickel(II) biosorption on Enteromorpha prolifera: optimization using response surface analysis. J Hazard Mater 152:778–788
Ahmadpour A, Tahmasbi M, Bastami TR, Besharati JA (2009) Rapid removal of cobalt ion from aqueous solutions by almond green hull. J Hazard Mater 166:925–930
Kaçan E, Kütahyalı C (2012) Adsorption of strontium from aqueous solution using activated carbon produced from textile sewage sludges. J Anal Appl Pyrol 97:149–157
Bhatnagar A, Minocha AK, Sillanpää M (2010) Adsorptive removal of cobalt from aqueous solution by utilizing lemon peel as biosorbent. Biochem Eng J 48:181–186
Montgomery DC (2001) Design and analysis of experiments. John Wiley & Sons, New York
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khani, M.H., Khamseh, A.G. Statistical analysis, equilibrium and dynamic study on the biosorption of strontium ions on Chlorella vulgaris. J Radioanal Nucl Chem 332, 3325–3334 (2023). https://doi.org/10.1007/s10967-023-09026-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09026-9