Abstract
A batch system was employed to investigate the biosorption of strontium (II) on Sargassum sp. The biosorption of strontium on Sargassum sp was studied with response surface methodology to determine the combined effect of temperature, initial metal ion concentration, biomass treatment, biosorbent dosage and pH. Under optimal conditions, the algae's biosorption capacity for strontium (initial pH 7.2, initial strontium concentration 300 mg/l for Mg-treated biomass and biosorbent dosage 0.1 g in 100 mL metal solution) was measured at 103.95 mg/g. In our analysis, equilibrium data were fitted to Langmuir and Freundlich isotherms. Results show that the best fit is provided by the Freundlich model. Biosorption dynamics analysis of the experimental data indicated that strontium (II) was absorbed into algal biomass in accordance with the pseudo-second-order kinetics model well.
Similar content being viewed by others
Introduction
Keeping radioactive substances contained is a crucial safety issue for nuclear industries1,2,3,4,5,6. The beta-emitting strontium (Sr)-90 radionuclide has a half-life of approximately 28 years, and it is produced by nuclear fission reactions. Water and wastewater industries use adsorption as one of the most important separation and purification processes to remove color, odor, and organic pollution7,8,9,10,11,12,13. There is a wide variety of microorganisms including bacteria, fungi, and algae that are used as metal adsorbents14,15,16,17,18,19. Also, the different synthesis procedures using biopolymer namely chitosan were applied to the adsorption of strontium from aqueous solutions20,21,22,23,24,25. Wang et al.26 used a carboxyl functionalized resin, i.e. SiMaC, prepared with in situ polymerization to adsorb strontium. They observed the maximum capacity of 142.5 mg/g at room temperature and fitting adsorption isotherms well with a pseudo-second-order (PSO) kinetics model. Qiu and co-workers27 studied the adsorption of strontium by living Saccharomyces cerevisiae. They concluded that bioaccumulation is vital during biosorption, and ion exchange is the main mechanism during separation compared to physical adsorption.
Among these microorganisms, Marine algae are more popular due to several parameters like low cost, their high metal biosorption capacity and their renewable nature28. Algal cell walls contain functional groups such as carboxyl, hydroxyl, and sulfhydryl, which allow them to attach metal ions from water29. Prabhu et al.30 studied the capability of the pteridophyte on the adsorption of Pb(II) from an aqueous solution. The biosorption capacity of 125 mg/g was observed with a removal efficiency of 99.93%. Yue and co-workers31 adsorbed Cd(II), Pb(II), and Cu(II) from an aqueous solution by using the amidoxime functionalized cellulose-based adsorbent. They observed that the Freundlich model predicted well the adsorption isotherm of both Cu(II) and Pb(II) ions, while the Langmuir model fitted well the adsorption isotherm of Cd(II).
As a tool, response surface methodology (RSM) can be active to investigate the interactions among two or more factors. Statistical and mathematical techniques are used in RSM to improve and optimize processes. In general, it involves three stages: (i) design and experiments, (ii) regression modeling, and (iii) optimization32,33,34,35,36,37.
In this study, Sargassum sp and chemically modified Sargassum sp were used for their ability to remove Sr from the solution. A feasibility study is conducted to investigate the use of adsorbent to remove Sr+2 from aqueous solutions by varying pH, temperature, initial strontium concentration, and brown algae dosage. We use the central composite design method to serve this purpose.
Materials and methods
Preparation of biosorbents
We collected samples of the marine algae Sargassum sp in the Persian Gulf along the coast of Qeshm, Iran. A combination of tap distilled water and water was used to wash the algae samples. This was done in order to remove sand as well as excess sodium and potassium ions. The samples were dried overnight at a maximum temperature of 55 °C, then ground to an average particle size of 0.7 mm to avoid degradation of binding sites38. Following loading with Mg2 + , the biomasses were soaked in 0.1 M Mg(NO3)2·6H2O (biomass concentration, 10 g/l) for 24 h under slow stirring. It was then necessary to wash the pretreated biomass several times with deionized water to reach a stable pH level for the washing solution, as well as to remove excess ions of magnesium from the biomasses. To use the Mg-pretreated biomasses in biosorption experiments, the biomasses were first dried overnight in an oven at 55 °C, and then natural algae as well as Mg-pretreated algae were utilized for the biosorption experiments.
Preparation of metal solutions
Strontium stock solutions containing 1000 ppm of strontium were prepared using analytical grade Sr(NO3)2 and the concentration of this solution was diluted based on the requirements. It should be noted that all solutions were prepared using deionized water. A dilute or concentrated solution of HNO3 and NaOH was used to adjust the pH of the strontium solutions before mixing them with the biosorbent. A grade of analytical chemicals was used throughout the experiment (Sigma Aldrich, Germany).
Analytical procedures
Energy dispersive X-ray spectroscopy (EDX, RONTECH, Germany) was utilized to analyze the properties of biosorbent earlier and subsequently its treatment with 0.1 M Mg (NO3)2·6 H2O and subsequently Sr2+ sorption.
A spectrophotometer based on atomic emission spectroscopy (ICP-AES, Optima, 7300DV, USA) and inductively coupled plasma were utilized to measure the levels of strontium dissolved in the solution. In this case, the ICP analysis was investigated at a wavelength of 460.733 nm.
Batch adsorption experiments
We performed sorption dynamics experiments on naturally occurring algae and on Mg-pretreated algae first to obtain the required contact time of sorption equilibrium experiments. In 250 ml Erlenmeyer flasks including 100 ml of 100 mg Sr/l Sr(NO3)2 solution, 0.1 gr of biomass was added. A shaker was used to agitate the flasks at 150 rpm and 25 °C for 24 h. A representative sample was selected at predetermined intervals over a period of time (2, 5, 15, 30, 45, 60, 90, 120, 150, 180 and 1440 min). ICP-AES spectrometry was then used to analyze the samples after dilution to determine the metal concentrations. It has been observed that the equilibrium of sorption dynamic tests was achieved after 150 min of contact. In batch adsorption experiments, magnesium ions were studied as surface modifiers of temperature, pH, biomass, biomass dosage and strontium concentration. A 250 ml Erlenmeyer flask containing 100 mL of Sr(NO3)2 solution with an initial concentration of strontium with different amounts of biomass was mixed. It is important to note that the flasks were shaken at a speed of 150 revolutions per minute for a period of 150 min. A diluted or concentrated solution of NaOH and HNO3 was mixed with the initial solution to adjust the pH before adding the biosorbent. In order to filter the strontium solution, Whatman filter paper was used. Following the filtering of the samples, the residual strontium ion concentration was measured. Comparing the amount of metal added to the biomass with the amount of metal in the supernatant, we calculated the amount of metal taken up by algae using Eq. 1:
In this equation, q (mg/g) at any given time t (min) is the adsorbed metal ions on the biosorbent, V (ml) is the volume of the metal-containing solution that is coming into contact with the biosorbent, C0 (mg/l) is the metal initial concentration, C (mg/l) at any given time t (min) is the concentration of metal in the solution, and Mads (g) is added biosorbent39.
Experimental design of biosorption testing
An experimental design was utilized to examine the effects of four numerical factors (biosorbent dosage, initial pH, initial metal ion concentration and temperature) at 5 levels and one categorical factor (biomass treatment) at 2 levels on the biosorption of strontium on sargassum sp and obtain optimal conditions. The Design-Expert software (version 7.1.4) was applied (Table 1) as part of the experimental design, All through testing, α was supposed to be 2.
To select the range of studied variables in Table 1, the temperature range was chosen to be around the ambient temperature with ± 15 due to proximity to possible industrial operating conditions. To investigate the effect of initial concentration, the range of real waste concentration and its simulation were considered. The widest range was chosen to investigate the effect of pH due to a lack of primary sense. To investigate the dose effect, the author’s previous experiences regarding biosorption processes were considered40,41,42,43,44,45,46,47.
Several techniques are applied to fit the polynomial model, including the central composite design (CCD)48,49,50. As a result of this method, as the mathematical model is empirical, modelling is simplified and the detailed reaction mechanism does not need to be known. In CCD, the number of trials is calculated as k + 2 k + n, where k represents the number of independent variables, 2 k represents the number of axial points, and n represents the number of center points51,52,53,54,55,56.
A linear or quadratic model can be used to relate the responses to the chosen factors in the optimization process. The quadratic model, along with the linear model, is written as;
where y is the response variable’s expected value; b0, bi, bii, bij are the model parameters; and xj and xi are the factors. For the empirical models, y1 and y2 represent the biosorption capacities of natural and modified algae, respectively, for the empirical models.
Confirmation experiment
A confirmatory experiment was conducted to check whether the models were valid by setting the experimental values at optimal levels expected by the models. Comparing biosorption capacity values obtained from the experiment with predictions from models, we checked whether they were within low or high confidence intervals.
Results and discussion
Statistical analysis
We used analysis of variance (ANOVA) results (see Table 2) to study the effect of different factors, e.g. biosorbent dosage, biomass treatment temperature, pH and metal ion concentration, on the response of the system. It is necessary to use this statistical device in order to examine the adequacy and significance of the model. As a measure of the degree to which the factors attempt to describe the variation in the data about the means, the Fischer variation ratio (F-value) can be considered. Analysis of variance F-values is calculated by dividing MS caused by model variation by MS caused by variance in error. As data have some variation around their mean value, it is generally accepted that the greater the F-value from the unity the more variation is the most acceptable57,58,59. Ideally, the F-value should be several times higher than the tabulated value. Additionally, p values less than 0.05 point to significant model terms. A P-value of less than 0.05 implies that the biosorption capacity model is statistically significant, as shown in Table 2. According to Table 2, the temperature is not significantly different from other factors such as CE (Mads, Type of biosorbent) and BE (C0, Type of biosorbent).
Fitting models
A CCD design was used to analyze 56 runs to obtain biosorption capacity. A modified quadratic model polynomial equation was fitted to the experimental results of the CCD using multiple regression analysis. Based on Eqs. (3) and (4), we calculate the biosorption capacity for strontium (II) ions in untreated and treated biomasses, respectively.
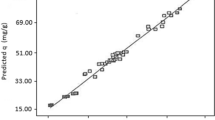
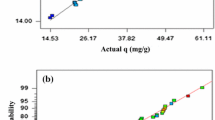
The above equations express the effects of various operating parameters such as pH, initial pollutant concentration, adsorbent dose, and also their interference effects in the adsorption rate of the studied adsorbents. We applied the polynomial models which could be considered as an approximation of the true functional relationship over relatively small regions of an independent variable space60. The predicted data as a function of actual data obtained from the experimental results is depicted in Fig. 1. A satisfactory correlation can be found between experimental data and predicted values based on the clustering around the diagonal line, which confirms the robustness of the model. As can be seen in Table 3, the modified quadratic model for biosorption capacity has high R2, adjusted R2, and adjusted R2 adj numbers, indicating that it can accurately represent the system under the given experimental conditions. To have adequate precision, it is important that the signal-to-noise ratio be higher than four, which is desirable. An increase in CV over 10 indicates a high variation in mean value, which prevents a satisfactory response model from developing32. Table 3 contains data that confirms these results. In Fig. 2, we show a normal probability plot and a studentized residual plot for the biosorption capacity of sargassum sp for strontium (II) ions. In addition to this, the residuals provide insight into the model's compliance with ANOVA assumptions, where the studentized residuals measure the difference between the predicted and actual values.
Process optimization using desirability functions
During the process, strontium (II) ions were absorbed by the biosorbent by maximizing biosorption capacity and finding optimal conditions. There are minimum and maximum levels that need to be specified in numerical optimization for each parameter. Multiple starting points in the design space section could highly improve the success rate of finding the best local maximum61. A 300 mg/l strontium (II) concentration, 0.1 g in 100 mL of biosorbent, and 7.2 pH were estimated to be the optimum values, respectively. The temperature does not affect optimization results, as shown in Table 2. Biosorbents with Mg(NO3)2·6H2O-treated biomass had the most metal uptake (103.95 mg/g) under optimum conditions (Mg-treated biomass).
Effect of Mg2+ as a pretreatment on biosorption capacity
Assuming other variables are kept at their central values, the interplay between the type of biomass and initial strontium (II) concentration is illustrated in Fig. 3. The adsorption capacities of both untreated sargassum sp and treated were assessed for initial strontium (II) concentration ions at different concentrations and it was found that Mg(NO3)2 treatment enhanced the adsorption capacity. At the sorption of initial strontium (II) concentration, the adsorption capacity for untreated sargassum sp increased from 17.03 to 58.59 mg/g, while this change for treated sargassum sp was from 17.38 to 68.84 mg/g with the increasing of the initial concentration in 50–300 mg/l. Consistent with these results, magnesium nitrate enhances adsorption capacity by acting as an effective biosorbent surface modifier. As shown in Table 2, initial strontium (II) concentration was significantly influenced by the type of biomass (BE) (p = 0.003). There was a clear uniform distribution of Sr on the surface of the biosorbent when element mapping was performed (Fig. 4a,b). A spot profile analysis of EDX spectra shows an enhancement of magnesium peak intensity compared with the spectra of biomass before magnesium treatment (Fig. 5a,b) and confirm the existence of strontium (Fig. 5c), signifying cations of metal are bonded to algae surfaces by negatively charged functional groups. Mg-treated biomass, strontium-loaded biomass, and untreated sargassum sp. were tested using EDX spectrometers as indicated in Table 4. Based on the data presented in this study, it appears that the Sr2+ ions have substituted the Mg2+ ions from the surface of the biosorbent and that the ion exchange mechanism participates in strontium biosorption. Because of the complexity of biomaterials and other mechanisms, e.g. chelation of metals and coordination, cations could adsorb on biomass and surface complex at the same time, to differ the degrees, in accordance with the biosorbent and solution chemistry62,63,64.
2D and 3D response plots
As shown in Figs. 6, 7, 8, the relationship between parameters is plotted in two and three dimensions according to their respective response plots. During the plot generation, two factors varied while the other factors remained constant in center values.
Metal ion transfer to sorbents is predominantly driven by the initial metal concentration, with higher metal concentrations resulting in greater metal uptake2,8,65. Similarly, in the present study, Sr2 + concentration increased from 50 to 300 mg/l increasing biosorption capacity by about 49.46 mg/g (Fig. 3). Sargassum sp's active sites will be surrounded by more strontium ions if the solution contains more strontium ions, therefore biosorption will occur more efficiently.
The response plot shown in Fig. 6 indicates how biomass dosage affects Sr concentration at 175 ppm and pH at 7.8. The biosorption capacity decreased from 83.07 to 35.73 mg/g for the Sr2+ ions, as the biosorbent dosage was increased from 0.1 to 0.5 g in 100 mL solution. In the case of higher dosages of biosorbent, the capacity of uptake is lower. It would be related to the unsaturation of biosorption sites through the biosorption reaction as well as the particle interaction, for instance, aggregation resulting from high biosorbent concentration66.
As shown in Fig. 7, pH and temperature (AD) affect strontium adsorption at the constant initial concentration (175 mg/L) and dosage (0.3 g). As pH increases above pH 7.2, sargassum sp’s biosorption capacity decreases with pH. Figure 7 shows that strontium (II) uptake was not significantly affected by biosorption temperature. Based on the ANOVA results, the solution temperature was not statistically significant (p-value = 0.303). Many adsorbate-adsorbent systems have also shown similar results8,67,68.
Plotting the perturbation plot (Fig. 8) reveals how each factor affects Mg-treated biomass at the central point in the design space, compared. This plot is based on an initial strontium (II) ion concentration of 175 mg/L, a biosorbent dose of 0.3 g, a temperature of 30 °C, and an initial pH of 7.8. The steep slope in biosorption capacity versus initial strontium (II) ion concentration indicates that biosorption capacity is very sensitive to those factors (see Fig. 8). It is evident from the relatively flat line of temperature that it is insensitive to changes in that factor.
Confirmatory experiment
A total of two experiments were conducted to validate the optimized model conditions based on values recommended by the models. The experimental results in Table 5 indicate good agreement between experimental results and the predicted values from the fitted correlations at a 95% confidence interval. The resulting experimental values matched the predicted values quite closely, proving that the model was valid. These conditions led to responses of 105.5 mg/g and 102.4 mg/g for biosorption capacity in the interval.
Kinetic study
As part of this research, second-order and first-order reaction rate kinetic models were applied to explain strontium sorption on sargassum sp. Equations (5) and (6) are linear forms of pseudo-second-order (PSO) and pseudo-first-order (PFO) kinetic models, respectively:
where qt (mg g−1) and qeq are the amount of strontium adsorbed at any given time t (min) and at equilibrium, respectively. k1 (min−1) show the equilibrium rate constant of pseudo-first-order and k2 (g mg-1 min-1) show the equilibrium rate constant of second-order adsorption. To determine k1 and qeq, one has to plot the log (qeq–qt) versus time in a linear plot. On the other side, t/qt versus time can be used to determine k2 and qeq.
Mg-treated biomass was subjected to two kinetics models to determine the biosorption of strontium ions at optimized conditions including C0 = 300 ppm, pH = 7.2, and Mads = 0.1 g. Results showed that the PFO model failed to explain all data obtained during contact time; therefore, coefficients with lower correlation were found (not presented). There have been reports of similar observations for a range of metals sorbing from aqueous solutions by biomass, proposing that it is a commonly observed feature in systems of sorption65,69. Because of the main limitation of the first-order equation of Lagergren, i.e. being normally appropriate for the initial rapid uptake, the sorption process takes about 20–30 min65. A plot of t/qt versus t for the PSO model can be seen in Fig. 9. Table 6 shows that theoretical qe(cal) values and experimental qe(exp) values are in good agreement. A correlation coefficient (R2) of 0.999 indicates that the present adsorption system is more well-defined by a PSO process. Because the PSO kinetic model provided the best fit, it can be expected that the rate-limiting step would be the chemisorption step65,70.
Isotherm at optimized condition
A comparison of Langmuir and Freundlich isotherms, classical sorption models, was performed to ascertain the relationship in the middle of adsorbed strontium ions on algal cells (qeq) and unadsorbed strontium ions in solution (Ceq). Equations (7) and (8) give the linear form of Langmuir and Freundlich isotherms, respectively:
where qeq (mg/g) is the equilibrium adsorbed amount, qm (mg/g) is the Langmuir constant, which represents the maximum adsorption capacity of monolayers, Ceq (mg/l) is the equilibrium concentration, and b (l/mg) is a measure of binding affinity based on the energy of adsorption71,72. A linear plot of 1/qeq versus 1/Ceq is used to determine the equilibrium parameters, i.e. qm and b. The constants k and n define the amount of adsorption and its intensity, respectively. 1/n and k can be obtained from plots of log qeq as a function of Ceq.
Design-Expert software calculated the isotherm of strontium sorption on Mg-treated biomass at constant optimized conditions such as pH = 7.2 and Mads = 0.1. Based on that data, the isotherms of strontium adsorption at 28 °C are provided in Table 7. Compared with Langmuir, the Freundlich model had better correlation coefficients (R2) for the adsorption equilibrium of strontium which implies the multilayer sorption of the strontium ions on the biosorbent73. As can be seen from Table 7, higher n values, i.e. more than unity, suggest satisfactory biosorption situations and the formation of stronger bonds between adsorbate and adsorbent67,74.
Conclusion
In this work, Sargassum sp. studied strontium uptake in intact and chemically modified forms. In the experimental design, CCD was used for the RSM, which was verified to be an effective strategy for trying to the impact of operating conditions on the process. It was found that a reduced quadratic model was able to expect the biosorption capacity. Based on the results of the ANOVA, the experimental and model data were significantly in agreement. Optimal biosorption conditions included magnesium-treated biomass, pH of 7.2, 0.1 g/100 mL biosorbent dose, and 300 mg/l strontium (II) ion concentration. In optimum conditions, Mg-treated biomass had a biosorption capacity of 103.95 mg/g for strontium (II) ions. It has been found that the Freundlich model is the most suitable for fitting the results which imply the multilayer sorption of the strontium ions on the biosorbent. Finally, the pseudo-second-order kinetics model described strontium (II) biosorption onto algal biomass well.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- b :
-
The Langmuir constant (l/mg)
- b 0 , b i and b ij :
-
Linear and quadratic interaction coefficients, i and j = 1–5
- C :
-
Metal ion concentration at any time (mg/l)
- C 0 :
-
Initial metal ion concentration(mg/l)
- C eq :
-
Residual metal ion concentration at equilibrium (mg/l)
- C.I. :
-
Confidence interval (−)
- C.V.:
-
Coefficient variation (−)
- k :
-
Adsorption capacity (−)
- n :
-
Biosorption intensity (−)
- q :
-
Amount of biosorbed metal per g of biosorbentat any time (mg/g)
- q eq :
-
The amount of biosorbent metal per unit weight of biosorbent at equilibrium(mg/g)
- q exp :
-
Experimental amount of biosorbent metal per unit weight of biosorbent at equilibrium (mg/g)
- q m :
-
Maximum capacity of biosorbent
- q pre :
-
The predicted amount of biosorbent metal per unit weight of biosorbent at equilibrium by software(mg/g)
- R 2 :
-
Correlation coefficient
- R 2 adj :
-
Adjusted correlation coefficient
- M ads :
-
Amount of biosorbent (g)
- t :
-
Time (min)
- x i :
-
The independent variable, i = 1–5
- y :
-
Response (−)
- V :
-
The volume of the solution (l)
- T :
-
Solution temperature (°C)
References
Ghorbanpour Khamseh, A. A., Amini, Y., Shademan, M. M. & Ghazanfari, V. Intensification of thorium biosorption onto protonated orange peel using the response surface methodology. Chem. Prod. Process Model. https://doi.org/10.1515/cppm-2022-0085 (2023).
Wang, L. et al. Adsorption–desorption of strontium from waters using aerobic granules. J. Taiwan Inst. Chem. Eng. 44, 454–457. https://doi.org/10.1016/j.jtice.2012.12.005 (2013).
Su, L. et al. Simultaneously and quantitatively analyze the heavy metals in Sargassum fusiforme by laser-induced breakdown spectroscopy. Food Chem. 338, 127797 (2021).
Liu, W. et al. Treatment of CrVI-containing Mg (OH)2 Nanowaste. Angew. Chem. 120, 5701–5704 (2008).
Chang, Y. et al. Adaptive tracking control for nonlinear system in pure-feedback form with prescribed performance and unknown hysteresis. IMA J. Math. Control. Inf. 39, 892–911 (2022).
Faress, F. et al. Phase equilibria simulation of biomaterial-hydrogen binary systems using a simple empirical correlation. Processes 11, 714 (2023).
Esmaeili Faraj, S. H., Nasr Esfahany, M. & Amini, Y. Modeling of biofiltration process for removal of vinyl chloride from an air stream by modified ottengraf model. J. Pet. Res. 24, 21–30. https://doi.org/10.22078/pr.2014.405 (2014).
Kaçan, E. & Kütahyalı, C. Adsorption of strontium from aqueous solution using activated carbon produced from textile sewage sludges. J. Anal. Appl. Pyrol. 97, 149–157. https://doi.org/10.1016/j.jaap.2012.06.006 (2012).
Yang, K. et al. Dysfunction of FadA-cAMP signalling decreases Aspergillus flavus resistance to antimicrobial natural preservative Perillaldehyde and AFB1 biosynthesis. Environ. Microbiol. 24, 1590–1607 (2022).
Pan, C. et al. Inhibitory effect of cinnamaldehyde on Fusarium solani and its application in postharvest preservation of sweet potato. Food Chem. 408, 135213 (2023).
Li, Y., Wang, H., Zhao, X. & Xu, N. Event-triggered adaptive tracking control for uncertain fractional-order nonstrict-feedback nonlinear systems via command filtering. Int. J. Robust Nonlinear Control 32, 7987–8011 (2022).
Aghel, B., Behaein, S. & Alobiad, F. CO2 capture from biogas by biomass-based adsorbents: A review. Fuel 328, 125276 (2022).
Aghel, B., Mansouri, S. & Varmira, K. Continuous purification of phosphoric acid in a microchannel. Iran. J. Chem. Chem. Eng. (IJCCE). 41(1), 79–87. https://doi.org/10.30492/IJCCE.2020.117915.3852 (2020).
Gabr, R. M., Hassan, S. H. A. & Shoreit, A. A. M. Biosorption of lead and nickel by living and non-living cells of Pseudomonas aeruginosa ASU 6a. Int. Biodeterior. Biodegrad. 62, 195–203. https://doi.org/10.1016/j.ibiod.2008.01.008 (2008).
Mukhopadhyay, M., Noronha, S. B. & Suraishkumar, G. K. Copper biosorption in a column of pretreated Aspergillus niger biomass. Chem. Eng. J. 144, 386–390. https://doi.org/10.1016/j.cej.2008.02.007 (2008).
Tabaraki, R., Nateghi, A. & Ahmady-Asbchin, S. Biosorption of lead (II) ions on Sargassum ilicifolium: Application of response surface methodology. Int. Biodeterior. Biodegrad. 93, 145–152. https://doi.org/10.1016/j.ibiod.2014.03.022 (2014).
Guo, Z. et al. Innovative and green utilization of zinc-bearing dust by hydrogen reduction: Recovery of zinc and lead, and synergetic preparation of Fe/C micro-electrolysis materials. Chem. Eng. J. 456, 141157 (2023).
Wang, Y. et al. Adaptive command filtered control for switched multi‐input multi‐output nonlinear systems with hysteresis inputs. Int. J. Adapt. Control Signal Process. 36(12), 3023–3042. https://doi.org/10.1002/acs.3501 (2022).
Amini, Y., Fattahi, M., Khorasheh, F. & Sahebdelfar, S. Neural network modeling the effect of oxygenate additives on the performance of Pt–Sn/γ-Al2 O3 catalyst in propane dehydrogenation. Appl. Petrochem. Res. 3, 47–54 (2013).
Hasan, S., Iasir, A. R. M., Ghosh, T. K., Sen Gupta, B. & Prelas, M. A. Characterization and Adsorption Behavior of Strontium from Aqueous Solutions onto Chitosan-Fuller’s Earth Beads. Healthcare. 7(1), 52. https://doi.org/10.3390/healthcare7010052 (2019).
Lupa, L., Voda, R. & Popa, A. Adsorption behavior of cesium and strontium onto chitosan impregnated with ionic liquid. Sep. Sci. Technol. 53, 1107–1115 (2018).
Yin, Y., Wang, J., Yang, X. & Li, W. Removal of strontium ions by immobilized Saccharomyces cerevisiae in magnetic chitosan microspheres. Nucl. Eng. Technol. 49, 172–177 (2017).
Li, T., He, F. & Dai, Y. Prussian blue analog caged in chitosan surface-decorated carbon nanotubes for removal cesium and strontium. J. Radioanal. Nucl. Chem. 310, 1139–1145 (2016).
Liang, Y. et al. Benzene decomposition by non-thermal plasma: A detailed mechanism study by synchrotron radiation photoionization mass spectrometry and theoretical calculations. J. Hazard. Mater. 420, 126584 (2021).
Chen, Z. et al. Pyrolysis temperature affects the physiochemical characteristics of lanthanum-modified biochar derived from orange peels: Insights into the mechanisms of tetracycline adsorption by spectroscopic analysis and theoretical calculations. Sci. Total Environ. 862, 160860 (2023).
Wang, S. et al. Synthesis of carboxyl group functionalized silica composite resin for strontium removal. Mater. Des. 185, 108224 (2020).
Qiu, L., Feng, J., Dai, Y. & Chang, S. Mechanisms of strontium’s adsorption by Saccharomyces cerevisiae: Contribution of surface and intracellular uptakes. Chemosphere 215, 15–24 (2019).
Liu, H. et al. Improvement of metal adsorption onto chitosan/Sargassum sp. composite sorbent by an innovative ion-imprint technology. Water Res. 45, 145–154. https://doi.org/10.1016/j.watres.2010.08.017 (2011).
Farooq, U., Kozinski, J. A., Khan, M. A. & Athar, M. Biosorption of heavy metal ions using wheat based biosorbents—a review of the recent literature. Biores. Technol. 101, 5043–5053. https://doi.org/10.1016/j.biortech.2010.02.030 (2010).
Prabhu, S., Srinikethan, G. & Hegde, S. Efficient biosorption of Pb (II) on Pteris vittata L. from aqueous solution using pulsed plate column technique. Sep. Sci. Technol. 55, 3089–3101 (2020).
Yue, Y. et al. Amidoxime functionalized low-cost cellulose-based adsorbent derived from waste cigarette filters for efficient heavy metal removal. J. Environ. Chem. Eng. 10, 107846 (2022).
Myers, R. H. & Montgomery, D. C. Response Surface Methodology: Process and Product Optimization Using Designed Experiments (Wiley, 2002).
Langroudi, L. O., Pahlavanzadeh, H. & Mousavi, S. M. Statistical evaluation of a liquid desiccant dehumidification system using RSM and theoretical study based on the effectiveness NTU model. J. Ind. Eng. Chem. 20, 2975–2983. https://doi.org/10.1016/j.jiec.2013.11.031 (2014).
Li, G. et al. Near-infrared responsive Z-scheme heterojunction with strong stability and ultra-high quantum efficiency constructed by lanthanide-doped glass. Appl. Catal. B 311, 121363 (2022).
Li, P. et al. Confidence interval based distributionally robust real-time dispatch considering wind power uncertainty. 8th Renewable Power Generation Conference (RPG 2019). 172, 6. https://doi.org/10.1049/cp.2019.0428 (2019).
Si, Z., Yang, M., Yu, Y. & Ding, T. Photovoltaic power forecast based on satellite images considering effects of solar position. Appl. Energy 302, 117514 (2021).
Wang, M., Yang, M., Fang, Z., Wang, M. & Wu, Q. A practical feeder planning model for urban distribution system. IEEE Trans. Power Syst. 38(2), 1297–1308. https://doi.org/10.1109/TPWRS.2022.3170933 (2022).
Amini, M. et al. Application of response surface methodology for optimization of lead biosorption in an aqueous solution by Aspergillus niger. J. Hazard. Mater. 154, 694–702. https://doi.org/10.1016/j.jhazmat.2007.10.114 (2008).
Demim, S. et al. Study of heavy metal removal from heavy metal mixture using the CCD method. J. Ind. Eng. Chem. 20, 512–520. https://doi.org/10.1016/j.jiec.2013.05.010 (2014).
Khani, M., Pahlavanzadeh, H. & Alizadeh, K. Biosorption of strontium from aqueous solution by fungus Aspergillus terreus. Environ. Sci. Pollut. Res. 19, 2408–2418 (2012).
Khani, M. H. Statistical analysis and isotherm study of uranium biosorption by Padina sp. algae biomass. Environ. Sci. Pollut. Res. 18, 790–799 (2011).
Khani, M. H. Uranium biosorption by Padina sp. algae biomass: Kinetics and thermodynamics. Environ. Sci. Pollut. Res. 18, 1593–1605 (2011).
Khani, M., Keshtkar, A., Ghannadi, M. & Pahlavanzadeh, H. Equilibrium, kinetic and thermodynamic study of the biosorption of uranium onto Cystoseria indica algae. J. Hazard. Mater. 150, 612–618 (2008).
Soleymani, F., Khani, M., Pahlavanzadeh, H. & Manteghian, M. Study of cobalt (II) biosorption on Sargassum sp. by experimental design methodology. Int. J. Environ. Sci. Technol. 12, 1907–1922 (2015).
Khani, M. Biosorption of strontium by Padina sp. algae biomass: Process optimisation and equilibrium study. Int. J. Environ. Technol. Manag. 16, 290–311 (2013).
Khani, M. Biosorption of strontium by a nonliving brown marine algae, Padina Sp. Sep. Sci. Technol. 47, 1886–1897 (2012).
Tan, J. et al. Screening of endocrine disrupting potential of surface waters via an affinity-based biosensor in a rural community in the Yellow River Basin, China. Environ. Sci. Technol. 56, 14350–14360 (2022).
Amini, Y. et al. Optimization of liquid-liquid extraction of calcium with a serpentine microfluidic device. Int. Commun. Heat Mass Transf. 140, 106551 (2023).
Marsousi, S., Karimi-Sabet, J., Moosavian, M. A. & Amini, Y. Liquid-liquid extraction of calcium using ionic liquids in spiral microfluidics. Chem. Eng. J. 356, 492–505 (2019).
Zhang, H., Zou, Q., Ju, Y., Song, C. & Chen, D. Distance-based support vector machine to predict DNA N6-methyladenine modification. Curr. Bioinform. 17, 473–482 (2022).
Montgomery, D. C. Design and Analysis of Experiments sixth. (Wiley, 2005).
Hashemipour, N. et al. Experimental and simulation investigation on separation of binary hydrocarbon mixture by thermogravitational column. J. Mol. Liq. 268, 791–806 (2018).
Jahromi, P. F., Karimi-Sabet, J., Amini, Y. & Fadaei, H. Pressure-driven liquid-liquid separation in Y-shaped microfluidic junctions. Chem. Eng. J. 328, 1075–1086 (2017).
Zhang, H., Zhao, X., Wang, H., Zong, G. & Xu, N. Hierarchical sliding-mode surface-based adaptive actor–critic optimal control for switched nonlinear systems with unknown perturbation. IEEE Trans. Neural Netw. Learn. Syst. 580, 756–774 (2022).
Cao, Y., Zhao, N., Xu, N., Zhao, X. & Alsaadi, F. E. Minimal-approximation-based adaptive event-triggered control of switched nonlinear systems with unknown control direction. Electronics 11, 3386 (2022).
Cao, C. et al. webTWAS: A resource for disease candidate susceptibility genes identified by transcriptome-wide association study. Nucleic Acids Res. 50, D1123–D1130 (2022).
Abdollahi, P., Karimi-Sabet, J., Moosavian, M. A. & Amini, Y. Microfluidic solvent extraction of calcium: Modeling and optimization of the process variables. Sep. Purif. Technol. 231, 115875 (2020).
Hashemipour, N. et al. Numerical study of n-heptane/benzene separation by thermal diffusion column. Chin. J. Chem. Eng. 27, 1745–1755 (2019).
Heydari, A., Alborzi, Z. S., Amini, Y. & Hassanvand, A. Configuration optimization of a renewable hybrid system including biogas generator, photovoltaic panel and wind turbine: Particle swarm optimization and genetic algorithms. Int. J. Modern Phys. C 34, 2350069 (2022).
Ebrahimi, B., Shojaosadati, S. A., Ranaie, S. O. & Mousavi, S. M. Optimization and evaluation of acetylcholine esterase immobilization on ceramic packing using response surface methodology. Process Biochem. 45, 81–87. https://doi.org/10.1016/j.procbio.2009.08.007 (2010).
Rastegar, S. O., Mousavi, S. M. & Shojaosadati, S. A. Cr and Ni recovery during bioleaching of dewatered metal-plating sludge using Acidithiobacillus ferrooxidans. Biores. Technol. 167, 61–68. https://doi.org/10.1016/j.biortech.2014.05.107 (2014).
Marešová, J. et al. Cobalt and strontium sorption by moss biosorbent: Modeling of single and binary metal systems. Desalination 266, 134–141. https://doi.org/10.1016/j.desal.2010.08.014 (2011).
Sheng, P. X., Ting, Y.-P., Chen, J. P. & Hong, L. Sorption of lead, copper, cadmium, zinc, and nickel by marine algal biomass: Characterization of biosorptive capacity and investigation of mechanisms. J. Colloid Interface Sci. 275, 131–141. https://doi.org/10.1016/j.jcis.2004.01.036 (2004).
Davis, T. A., Volesky, B. & Mucci, A. A review of the biochemistry of heavy metal biosorption by brown algae. Water Res. 37, 4311–4330. https://doi.org/10.1016/S0043-1354(03)00293-8 (2003).
Ngwenya, N. & Chirwa, E. M. N. Single and binary component sorption of the fission products Sr2+, Cs+ and Co2+ from aqueous solutions onto sulphate reducing bacteria. Miner. Eng. 23, 463–470. https://doi.org/10.1016/j.mineng.2009.11.006 (2010).
Özer, A., Gürbüz, G., Çalimli, A. & Körbahti, B. K. Biosorption of copper(II) ions on Enteromorpha prolifera: Application of response surface methodology (RSM). Chem. Eng. J. 146, 377–387. https://doi.org/10.1016/j.cej.2008.06.041 (2009).
Özer, A., Gürbüz, G., Çalimli, A. & Körbahti, B. K. Investigation of nickel(II) biosorption on Enteromorpha prolifera: Optimization using response surface analysis. J. Hazard. Mater. 152, 778–788. https://doi.org/10.1016/j.jhazmat.2007.07.088 (2008).
Ahmadpour, A., Tahmasbi, M., Bastami, T. R. & Besharati, J. A. Rapid removal of cobalt ion from aqueous solutions by almond green hull. J. Hazard. Mater. 166, 925–930. https://doi.org/10.1016/j.jhazmat.2008.11.103 (2009).
Bulgariu, D. & Bulgariu, L. Equilibrium and kinetics studies of heavy metal ions biosorption on green algae waste biomass. Bioresource Technology. 103(1), 489–493 (2012).
Pahlavanzadeh, H., Keshtkar, A. R., Safdari, J. & Abadi, Z. Biosorption of nickel(II) from aqueous solution by brown algae: Equilibrium, dynamic and thermodynamic studies. J. Hazard. Mater. 175, 304–310. https://doi.org/10.1016/j.jhazmat.2009.10.004 (2010).
Bhatnagar, A., Minocha, A. K. & Sillanpää, M. Adsorptive removal of cobalt from aqueous solution by utilizing lemon peel as biosorbent. Biochem. Eng. J. 48, 181–186. https://doi.org/10.1016/j.bej.2009.10.005 (2010).
Montgomery, D. C. Design and Analysis of Experiments (Wiley, 2001).
Singh, R. et al. Biosorption optimization of lead(II), cadmium(II) and copper(II) using response surface methodology and applicability in isotherms and thermodynamics modeling. J. Hazard. Mater. 174, 623–634. https://doi.org/10.1016/j.jhazmat.2009.09.097 (2010).
Dahiya, S., Tripathi, R. M. & Hegde, A. G. Biosorption of heavy metals and radionuclide from aqueous solutions by pre-treated arca shell biomass. J. Hazard. Mater. 150, 376–386. https://doi.org/10.1016/j.jhazmat.2007.04.134 (2008).
Author information
Authors and Affiliations
Contributions
F.S: wrote the main manuscript text, reviewed the manuscript, prepared figures and tables M.H.K: wrote the main manuscript text, reviewed the manuscript, prepared figures and tables H.P: reviewed the manuscript, prepared figures and tables Y.A: wrote the main manuscript text, reviewed the manuscript, prepared tables.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Soleymani, F., Khani, M.H., Pahlevanzadeh, H. et al. Intensification of strontium (II) ion biosorption on Sargassum sp via response surface methodology. Sci Rep 13, 5403 (2023). https://doi.org/10.1038/s41598-023-32532-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-32532-5
- Springer Nature Limited
This article is cited by
-
Bacteria were unable to tolerate the radionuclides, while the halophilic fungi tolerated and efficiently remediated them
International Journal of Environmental Science and Technology (2024)
-
Solvent extraction of uranium from leach liquor using dibutyl carbitol: optimization of parameters by response surface methodology
Journal of Radioanalytical and Nuclear Chemistry (2024)
-
Investigation of kinetic, isotherm and adsorption efficacy of thorium by orange peel immobilized on calcium alginate
Scientific Reports (2023)
-
Ce(III) and La(III) ions adsorption using Amberlite XAD-7 resin impregnated with DEHPA extractant: response surface methodology, isotherm and kinetic study
Scientific Reports (2023)
-
Statistical analysis, equilibrium and dynamic study on the biosorption of strontium ions on Chlorella vulgaris
Journal of Radioanalytical and Nuclear Chemistry (2023)