Abstract
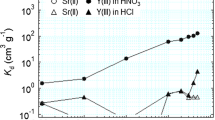
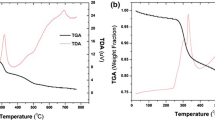
For separating americium from lanthanides, a macroporous silica-based polymeric adsorbent (HDEHP/SiO2-P) was prepared in this study. Using this adsorbent, the adsorption characteristics of 241Am(III) and lanthanides were investigated in HNO3 solutions. The obtained results displayed that heavy lanthanides had greater adsorption towards HDEHP/SiO2-P than Ce(III) and 241Am(III). The adsorption behavior of 241Am(III) was found to be similar with Ce(III) in 0.1–0.3 M HNO3 solutions. The adsorption kinetics of Gd(III) and Ce(III) reached equilibrium within 2 h and fitted well with the pseudo-second-order kinetic model.








Similar content being viewed by others
References
Yamaji K (2015) Issues of HLW disposal in Japan. In: Nakajima K (ed) Nuclear back-end and transmutation technology for waste disposal: beyond the Fukushima accident. Springer, Tokyo, pp. 279–287
Van Hecke K, Goethals P (2006) Research on advanced aqueous reprocessing of spent nuclear fuel: literature study. Open report of the Belgian nuclear research centre. SCK CEN, BLG-1030
Kolarik Z (1998) Current european research on the separation of actinides from high-level radioactive wastes. J Nucl Fuel Cycle Environ 5:21–23
Madic C (2001) Overview of the hydrometallurgical and pyrometallurgical processes studied world-wide for the partitioning of high active nuclear wastes. In: Proceedings Symposium NUCEF2001, Tokai, Japan, October 31–November 2, p 27
Bourg S, Hill C, Caravaca C, Rhodes C, Ekberg C, Taylor R, Geist A, Modolo G, Cassayre L, Malmbeck R, Harrison M, de Angelis G, Espartero A, Bouvet S, Ouvrier N (2011) ACSEPT—Partitioning technologies and actinide science: towards pilot facilities in Europe. Nucl Eng Des 241(9):3427–3435
Malmbeck R, Nourry C, Ougier M, Souček P, Glatz J, Kato T, Koyama T (2011) Advanced fuel cycle options. Energy Procedia 7:93–102
Herbst RS, Law JD, Todd TA, Romanovskiy VN, Babain VA, Esimantovskiy VM, Smirnov IV, Zaitsev BN (2002) Universal solvent extraction (UNEX) flowsheet testing for the removal of cesium, strontium, and actinide elements from radioactive, acidic dissolved calcine waste. Solvent Extr Ion Exch 20:429–445
Koma Y, Watanabe M, Nemoto S, Tanaka Y (1998) A counter current experiment for the separation of trivalent actinides and lanthanides by the SETFICS process. Solvent Extr Ion Exch 16:1357–1367
Zhang A, Wei Y, Hoshi H, Kumagai M, Kamiya M, Koyama T (2005) Resistance properties of a macroporous silica-based N, N, N′, N′-tetraoctyl-3- oxapentane-1, 5-diamide-impregnated polymeric adsorption material against nitric acid, temperature and γ-irradiation. Radiat Phys Chem 72:669–678
Ossola A, Macerata E, Tinonin DA, Faroldi F, Giola M, Mariani M, Casnati A (2016) Radiolytic degradation of a new diglycol-diamide ligand for actinide and lanthanide co-extraction from spent nuclear fuel. Radiat Phys Chem 124:246–251
Wei YZ, Zhang AY, Kumagai M, Watanabe M, Hayashi N (2004) Development of the MAREC process for HLLW partitioning using a novel silica-based CMPO extraction resin. J Nucl Sci Technol 41:315–322
Wei YZ, Kumagai M, Takashima Y, Modolo G, Odoj R (2000) Studies on the separation of minor actinides from high-level wastes by extraction chromatography using novel silica-based extraction resins. Nucl Technol 132:413–423
Zhang AY, Kuraoka E, Hoshi H, Kumagai M (2004) Synthesis of two novel macroporous silica-based impregnated polymeric composites and their application in highly active liquid waste partitioning by extraction chromatography. J Chromatogr A 1061:175–182
Ruiqin L, Yuezhou W, Tozawa D, Yuanlai X, Usuda S, Yamazaki H, Ishii K, Sano Y, Koma Y (2011) Evaluation study on properties of a macroporous silica-based CMPO extraction resin to be used forminor actinides separation from high level liquid waste. Nucl Sci Technol 22:18–24
Zhang AY, Hu QH, Wang WH, Kuraoka E (2008) Application of a macroporous silica-based CMPO-impregnated polymeric composite in group partitioning of long-lived minor actinides from highly active liquid by extraction chromatography. Ind Eng Chem Res 47:6158–6165
Alonso JIG, Sena F, Arbore P, Betti M, Koch L (1995) Determination of fission products and actinides in spent nuclear fuels by isotope dilution ion chromatography inductively coupled plasma mass spectrometry. J Anal At Spectrom 10:381–393
Hoshi H, Wei Y, Kumagai M, Asakura H, Uchiyama G (2002) Elemental groups separation for high-level waste partitioning using a novel silica-based CMPO extraction-resin. J Nucl Sci Technol 39:874–877
Zhang A, Wei Y, Hoshi H, Kumagai M (2005) Chromatographic separation of strontium (II) from a nitric acid solution containing some typically simulated elements by a novel silica-based TODGA impregnated polymeric composite in the MAREC process. Solvent Extr Ion Exch 23:231–247
Xu Y, Kim S-Y, Usuda S, Wei Y, Ishii K (2013) Adsorption and desorption behavior of tetravalent zirconium onto a silica-based macroporous TODGA adsorbent in HNO3 solution. J Radioanal Nucl Chem 297:91–96
Ning S, Wang X, Liu R, Wei Y, He L, Tang F (2015) Evaluation of Me2-CA-BTP/SiO2-P adsorbent for the separation of minor actinides from simulated HLLW. J Radioanal Nucl Chem 303:2011–2017
Lewis FW, Harwood LM, Hudson MJ, Distler P, John J, Stamberg K, Núñez A, Galán H, Espartero AG (2012) Synthesis and evaluation of lipophilic BTBP ligands for An/Ln separation in nuclear waste treatment: the effect of alkyl substitution on extraction properties and implications for ligand design. Eur J Org Chem 8:1509–1519
Xiao CL, Wang CZ, Yuan LY, Li B, He H, Wang SA, Zhao YL, Chai ZF, Shi WQ (2014) Excellent selectivity for actinides with a tetradentate 2, 9-diamide-1, 10-phenanthroline ligand in highly acidic solution: a hard–soft donor combined strategy. Inorg Chem 53:1712–1720
Trumm S, Geist A, Panak PJ, Fanghänel T (2011) An improved hydrolytically-stable bis-triazinyl-pyridine (BTP) for selective actinide extraction. Solvent Ext Ion Exch 29:213–329
Nilsson M, Nash KL (2007) Review article: a review of the development and operational characteristics of the TALSPEAK process. Solvent Extr Ion Exch 25:665–701
Lumetta GJ, Neiner D, Sinkov SI, Carter JC, Braley JC, Latesky S, Gelis AV, Tkac P, Vandegrift GF (2011) Combining neutral and acidic extractants for recovering transuranic elements from nuclear fuel. In: Proceedings of the 19th International Solvent Extraction Conference, Gecamin Ltd, Santiago, Chile, Chap. 3, Paper No. 68
Koma Y, Watanabe S, Sano Y, Asakura T, Morita Y (2008) Extraction chromatography for Am and Cm recovery in engineering scale. In: Proceedings ATALANTE 2008: nuclear fuel cycles for a sustainable future, Montpellier, France, 19–23 May 2008
McDowell WJ, Perdue PT (1976) Purification of di(2-ethylhexyl)phosphoric acid. J Inorg Nucl Chem 38:2127–2129
Nash KL (1993) A review of the basic chemistry and recent developments in trivalent f-elements separations. Solvent Extr Ion Exch 11:729–768
Xu Y, Wei Y, Liu R, Usuda S, Ishii K, Yamazaki H (2011) Adsorption characteristics of trivalent rare earths and chemical stability of a silica-based macroporous todga adsorbent in HNO3 solution. J Nucl Sci Technol 48:1223–1229
Yoshihiro N, Kadono J, Nishiuchi S, Yamamoto S, Tanabe T, Miyake H (2006) Hydrogen-desorbing behavior of the hydrides in 15 rare earth elements measured by temperature swing column chromatography. J Alloys Compd 408:355–358
O’Shannessy DJ, Winzor DJ (1996) Interpretation of deviations from pseudo-first-order kinetic behavior in the characterization of ligand binding by biosensor technology. Anal Biochem 236:275–283
Liu Y (2008) New insights into pseudo-second-order kinetic equation for adsorption. Colloids Surf A 320:275–278
Li ZC, Fan HT, Zhang Y, Chen MX, Yu ZY, Cao XQ, Sun T (2011) Cd(II)-imprinted polymer sorbents prepared by combination of surface imprinting technique with hydrothermal assisted sol–gel process for selective removal of cadmium(II) from aqueous solution. Chem Eng J 171(2):703–710
Langmuir I (1917) The constitution and fundamental properties of solids and liquids. II. Liquids. 1. J Am Chem Soc 39:1848–1906
LeVan MD, Vermeulen T (1981) Binary Langmuir and Freundlich isotherms for ideal adsorbed solutions. J Phys Chem 85:3247–32501
Yuan YX, Liu JS, Zhou BX, Yao SY, Li HM, Xu WX (2010) Synthesis of coated solvent impregnated resin for the adsorption of indium (III). Hydrometallurgy 101:148–155
El-Kamash AM, Awwad NS, El-Sayed AA (2005) Sorption of uranium and thorium ions from nitric acid solution using HDEHP-impregnated activated carbon. Arab J Nucl Sci Appl 38(1):43–51
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 91126006 and Grant No. 11305102) and Major Science and Technology Program for Water Pollution Control and Treatment (Grant No. 2015ZX07406006-06).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shu, Q., Khayambashi, A., Zou, Q. et al. Studies on adsorption and separation characteristics of americium and lanthanides using a silica-based macroporous bi(2-ethylhexyl) phosphoric acid (HDEHP) adsorbent. J Radioanal Nucl Chem 313, 29–37 (2017). https://doi.org/10.1007/s10967-017-5293-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-017-5293-z