Abstract
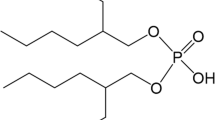
To separate Y(III) from a Sr(II)–Y(II) mixture, two silica-based adsorbents, CMPO/SiO2-P and (CMPO + Dodec)/SiO2-P were prepared by impregnating octyl(phenyl)-N,N-diisobutylcarbamoyl methylphosphine oxide (CMPO) extractant and a molecule modifier (1-Dodecanol) into macroporous silica/polymer composite support (SiO2-P). The Adsorbents showed high adsorption affinity to Y(III) and weak adsorption affinity to Sr(II) in HNO3 solution. The amount of adsorbed Y(III) increased with the HNO3 in the solution. The amount of adsorbed Y(III) increased with contact time and reached equilibrium within 30 min. The results showed that a pseudo-second-order kinetic model with a high correlation coefficient described the adsorption process better than other kinetic models. Successful Y(III) separation from Sr(II)–Y(III) mixture in HNO3 solution was achieved.







Similar content being viewed by others
References
Zhamg A, Kuraoka E, Kumagai M (2007) Development of the chromatographic partitioning of cesium and strontium utilizing two macroporous silica-based calix[4]arene-crown and amide impregnated polymeric composites: PREC partitioning process. J Chromatogr A 1157(1):85–95
Wu Y, Kim SY, Tozawa D, Ito T, Tada T, Hitomi K, Kuraoka E, Yamazaki H, Ishii K (2012) Equilibrium and kinetic studies of selective adsorption and separation for strontium using DtBuCH18C6 loaded resin. J Nucl Sci Technol 49(3):320–327
Chakravarty R, Pandey U, Manolkar RB, Dash A, Venkatesh M, Pillai MRA (2008) Development of an electrochemical 90Sr–90Y generator for separation of 90Y suitable for targeted therapy. Nucl Med Biol 35(2):245–253
Lee JS, Park UJ, Son KJ, Han HS (2009) One column operation for 90Sr/90Y separation by using a functionalized-silica. Appl Radiat Isot 67(7):1332–1335
Vanura P, Makrlik E (2002) Separation of microamounts of yttrium from strontium by using nitrobenzenesolution of sodium dicarbollylcobaltate in the presence of 18-crown-6. J Radioanal Nucl Chem 251(3):499–501
Schulz LA, Bray LA (1987) Solvent extraction recovery of byproduct 137Cs and 90Sr from HNO3 solutions-A technology review and assessment. Sep Sci Technol 22(2-3):191–214
Horwitz EP, Dietz ML, Fisher DE (1991) SREX: a newprocess for the extraction and recovery of strontium from acidic nuclear waste streams. Solv Extr Ion Exch 9(1):1–25
Pais J, Selucky P, Kyrs M (1976) Extraction of alkali metals into nitrobenzene in the presence of univalent polyhedral borate anions. J Inorg Nucl Chem 38(7):1376–1378
Wheelwright EJ (1970) A generic ion-exchange process for the recovery and purification of valuable elements from the nuclear industry. In: Conference proceedings of ion exchange in the process industries, 16–18 July 1969, London, United Kingdom Society of Chemical Industry, pp 202–208
Horwitz EP, Dietz ML, Fisher DE (1991) Separation and preconcentration of strontium from biological, environmental, and nuclear waste samples by extraction chromatography using a crown ether. Anal Chem 63(5):522–525
Lumetta GJ, Wester DW, Morrey JR, Wagner MJ (1993) Preliminary evaluation of chromatographic techniques for the separation of radionuclides from high-level radioactive waste. Solv Extr Ion Exch 11(4):663–682
Xu Y, Kim S-Y, Ito T, Nakazawa K, Funaki Y, Tada T, Hitomi K, Ishii K (2012) Adsorption and separation behavior of yttrium and strontium in nitric acid solution by extraction chromatography using a macroporous silica-based adsorbent. J Chromatogr A 1263:28–33
Turanov AN, Karandashev VK, Yarkevich AN, Safronova ZV, Kharitonov AV, Radygina NI, Fedoseev AM (2004) Extraction of U(VI), Th(IV), Pu(IV), Am(III), and rare-earth elements from nitric acid solutions with diphenyl-(dialkylcarbamoylmethyl)phosphine oxides substituted in the methylene bridge. Radiochemistry 46:461–467
Ho YS, Mckay G (1999) Pseudo-second order model for sorption process. Process Biochem 34(5):451–465
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number 16H02444.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kawamura, T., Ito, T. & Kim, SY. Adsorption and separation behavior of strontium and yttrium using a silica-based CMPO adsorbent. J Radioanal Nucl Chem 320, 9–14 (2019). https://doi.org/10.1007/s10967-019-06446-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-019-06446-4