Abstract
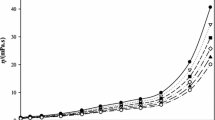
We have conducted the first investigation of the thermophysical and transport properties of N-hexyl pyridinium nitrate [HPy][NO3], a room-temperature ionic liquid, in combination with water and the organic solvents, ethanol, and acetonitrile in the form of binary mixtures, at 298.15 K and 101 kPa. The densities, viscosities, and refractive indices of the binary mixtures were measured, and the experimental values were used to calculate the derived properties. To examine the volumetric properties of the binary mixtures, the apparent molar volumes were calculated from experimental densities and correlated using both the Redlich–Mayer and Pitzer models. It was found that the dielectric constant and polarity of the solvents had significant effects on the ion–solvent interactions. The viscosities and refractive indices of the mixtures are reported; the B-coefficient of the Jones–Dole equation, and the molar refractive indices determined using the Lorentz–Lorenz equation were calculated. Values of the standard deviations between the calculated and experimental data were determined using the linear least-squares method. Finally, the results were used to shed light on the structure making/breaking effect of ions in the binary mixtures.




Similar content being viewed by others
References
Gabriel, S., Weiner, J.: Ueber einige abkömmlinge des propylamins. Ber. Dtsch. Chem. Ges. 21(2), 2669–2679 (1888)
Walden, P.: Molecular weights and electrical conductivity of several fused salts. Bull. Acad. Imper. Sci. (St. Petersburg) 1800 (1914)
Chum, H.L., Koch, V., Miller, L., Osteryoung, R.: Electrochemical scrutiny of organometallic iron complexes and hexamethylbenzene in a room temperature molten salt. J. Am. Chem. Soc. 97(11), 3264–3265 (1975)
Gale, R., Osteryoung, R.: Potentiometric investigation of dialuminum heptachloride formation in aluminum chloride-1-butylpyridinium chloride mixtures. Inorg. Chem. 18(6), 1603–1605 (1979)
Wilkes, J.S., Levisky, J.A., Wilson, R.A., Hussey, C.L.: Dialkylimidazolium chloroaluminate melts: a new class of room-temperature ionic liquids for electrochemistry, spectroscopy and synthesis. Inorg. Chem. 21(3), 1263–1264 (1982)
Wu, B., Reddy, R.G., Rogers, R.D.: Novel ionic liquid thermal storage for solar thermal electric power systems. In: International Solar Energy Conference 2001, pp. 445–451. American Society of Mechanical Engineers (2001)
Lapkin, A.A., Plucinski, P.K., Cutler, M.: Comparative assessment of technologies for extraction of artemisinin. J. Nat. Prod. 69(11), 1653–1664 (2006)
Jayakumar, M., Venkatesan, K., Srinivasan, T.: Electrochemical behavior of fission palladium in 1-butyl-3-methylimidazolium chloride. Electrochim. Acta 52(24), 7121–7127 (2007)
Barghi, S., Adibi, M., Rashtchian, D.: An experimental study on permeability, diffusivity, and selectivity of CO2 and CH4 through [bmim][PF6] ionic liquid supported on an alumina membrane: investigation of temperature fluctuations effects. J. Membrane Sci. 362(1–2), 346–352 (2010)
Earle, M.J., Esperança, J.M., Gilea, M.A., Lopes, J.N.C., Rebelo, L.P., Magee, J.W., Seddon, K.R., Widegren, J.A.: The distillation and volatility of ionic liquids. Nature 439(7078), 831–834 (2006)
Armand, M., Endres, F., MacFarlane, D.R., Ohno, H., Scrosati, B.: Ionic-liquid materials for the electrochemical challenges of the future. In: Materials for Sustainable Energy: A Collection of Peer-reviewed Research and Review Articles from Nature Publishing Group. pp. 129–137. World Scientific, Singapore (2011)
Kubisa, P.: Ionic liquids as solvents for polymerization processes—progress and challenges. Prog. Polym. Sci. 34(12), 1333–1347 (2009)
Kubota, F., Shimobori, Y., Baba, Y., Koyanagi, Y., Shimojo, K., Kamiya, N., Goto, M.: Application of ionic liquids to extraction separation of rare earth metals with an effective diglycol amic acid extractant. J. Chem. Eng. Jpn. 1102280146–1102280146 (2011)
Plechkova, N.V., Seddon, K.R.: Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 37(1), 123–150 (2008)
Rogers, R.D., Seddon, K.R.: Ionic liquids–solvents of the future? Science 302(5646), 792–793 (2003)
Wasserscheid, P., van Hal, R., Bösmann, A.: 1-n-Butyl-3-methylimidazolium ([bmim]) octylsulfate—an even ‘greener’ionic liquid. Green Chem. 4(4), 400–404 (2002)
Zhu, S., Wu, Y., Chen, Q., Yu, Z., Wang, C., Jin, S., Ding, Y., Wu, G.: Dissolution of cellulose with ionic liquids and its application: a mini-review. Green Chem. 8(4), 325–327 (2006)
Marrucho, I., Branco, L., Rebelo, L.: Ionic liquids in pharmaceutical applications. Annu. Rev. Chem. Biomol. 5, 527–546 (2014)
Stoimenovski, J., MacFarlane, D.R., Bica, K., Rogers, R.D.: Crystalline vs. ionic liquid salt forms of active pharmaceutical ingredients: a position paper. Pharmaceut. Res. 27(4), 521–526 (2010)
Weaver, K.D., Kim, H.J., Sun, J., MacFarlane, D.R., Elliott, G.D.: Cyto-toxicity and biocompatibility of a family of choline phosphate ionic liquids designed for pharmaceutical applications. Green Chem. 12(3), 507–513 (2010)
Ha, S.H., Menchavez, R.N., Koo, Y.-M.: Reprocessing of spent nuclear waste using ionic liquids. Korean J. Chem. Eng. 27(5), 1360–1365 (2010)
Sun, X., Luo, H., Dai, S.: Ionic liquids-based extraction: a promising strategy for the advanced nuclear fuel cycle. Chem. Rev. 112(4), 2100–2128 (2012)
Audic, N., Clavier, H., Mauduit, M., Guillemin, J.-C.: An ionic liquid-supported ruthenium carbene complex: a robust and recyclable catalyst for ring-closing olefin metathesis in ionic liquids. J. Am. Chem. Soc. 125(31), 9248–9249 (2003)
Dupont, D., Binnemans, K.: Rare-earth recycling using a functionalized ionic liquid for the selective dissolution and revalorization of Y2O3: Eu3+ from lamp phosphor waste. Green Chem. 17(2), 856–868 (2015)
Yang, F., Kubota, F., Baba, Y., Kamiya, N., Goto, M.: Selective extraction and recovery of rare earth metals from phosphor powders in waste fluorescent lamps using an ionic liquid system. J. Hazard. Mater. 254, 79–88 (2013)
Wang, P., Zakeeruddin, S.M., Comte, P., Exnar, I., Grätzel, M.: Gelation of ionic liquid-based electrolytes with silica nanoparticles for quasi-solid-state dye-sensitized solar cells. J. Am. Chem. Soc. 125(5), 1166–1167 (2003)
Wang, P., Zakeeruddin, S.M., Exnar, I., Grätzel, M.: High efficiency dye-sensitized nanocrystalline solar cells based on ionic liquid polymer gel electrolyte. Chem. Commun. 24, 2972–2973 (2002)
Wang, P., Zakeeruddin, S.M., Moser, J.-E., Grätzel, M.: A new ionic liquid electrolyte enhances the conversion efficiency of dye-sensitized solar cells. J. Phys. Chem. B 107(48), 13280–13285 (2003)
Ue, M., Takeda, M., Toriumi, A., Kominato, A., Hagiwara, R., Ito, Y.: Application of low-viscosity ionic liquid to the electrolyte of double-layer capacitors. J. Electrochem. Soc. 150(4), A499 (2003)
Fujii, K., Fujimori, T., Takamuku, T., Kanzaki, R., Umebayashi, Y., Ishiguro, S.-I.: Conformational equilibrium of bis (trifluoromethanesulfonyl) imide anion of a room-temperature ionic liquid: Raman spectroscopic study and DFT calculations. J. Phys. Chem. B 110(16), 8179–8183 (2006)
Hao, Y., Peng, J., Hu, S., Li, J., Zhai, M.: Thermal decomposition of allyl-imidazolium-based ionic liquid studied by TGA–MS analysis and DFT calculations. Thermochim. Acta 501(1–2), 78–83 (2010)
Jiang, W., Wang, Y., Voth, G.A.: Molecular dynamics simulation of nanostructural organization in ionic liquid/water mixtures. J. Phys. Chem. B 111(18), 4812–4818 (2007)
Kresse, G., Hafner, J.: Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47(1), 558 (1993)
Morrow, T.I., Maginn, E.J.: Molecular dynamics study of the ionic liquid 1-n-butyl-3-methylimidazolium hexafluorophosphate. J. Phys. Chem. B 106(49), 12807–12813 (2002)
Canongia Lopes, J.N., Deschamps, J., Pádua, A.A.: Modeling ionic liquids using a systematic all-atom force field. J. Phys. Chem. B 108(6), 2038–2047 (2004)
Canongia Lopes, J.N., Pádua, A.A.: Molecular force field for ionic liquids composed of triflate or bistriflylimide anions. J. Phys. Chem. B 108(43), 16893–16898 (2004)
Mirarabrazi, M., Stolarska, O., Smiglak, M., Robelin, C.: Solid–liquid equilibria for a pyrrolidinium-based common-cation ternary ionic liquid system, and for a pyridinium-based ternary reciprocal ionic liquid system: an experimental study and a thermodynamic model. Phys. Chem. Chem. Phys. 20(1), 637–657 (2018)
Dai, J., Wang, H., Li, Y., Xiu, Z.-L.: Imidazolium ionic liquids-based salting-out extraction of 2, 3-butanediol from fermentation broths. Process Biochem. 71, 175–181 (2018)
Ding, H., Ye, W., Wang, Y., Wang, X., Li, L., Liu, D., Gui, J., Song, C., Ji, N.: Process intensification of transesterification for biodiesel production from palm oil: microwave irradiation on transesterification reaction catalyzed by acidic imidazolium ionic liquids. Energy 144, 957–967 (2018)
Heitz, M.P., Rupp, J.W.: Determining mushroom tyrosinase inhibition by imidazolium ionic liquids: a spectroscopic and molecular docking study. Int. J. Biol. Macromol. 107, 1971–1981 (2018)
Yadav, A., Guha, A., Pandey, A., Pal, M., Trivedi, S., Pandey, S.: Densities and dynamic viscosities of ionic liquids having 1-butyl-3-methylimidazolium cation with different anions and bis (trifluoromethylsulfonyl) imide anion with different cations in the temperature range (283.15 to 363.15) K. J. Chem. Thermodyn. 116, 67–75 (2018)
Halayqa, M., Pobudkowska, A., Domańska, U., Zawadzki, M.: Studying of drug solubility in water and alcohols using drug-ammonium ionic liquid-compounds. Eur. J. Pharm. Sci. 111, 270–277 (2018)
Harris, M.A., Heres, M.F., Coote, J., Wenda, A., Strehmel, V., Stein, G.E., Sangoro, J.: Ion transport and interfacial dynamics in disordered block copolymers of ammonium-based polymerized ionic liquids. Macromolecules 51(9), 3477–3486 (2018)
Lepre, L., Pison, L., Siqueira, L., Ando, R., Gomes, M.C.: Improvement of carbon dioxide absorption by mixing poly(ethylene glycol) dimethyl ether with ammonium-based ionic liquids. Sep. Purif. Technol. 196, 10–19 (2018)
Zafarani-Moattar, M.T., Shekaari, H., Hashemzadeh, T.: Effect of temperature and molar mass of polymer on liquid–liquid equilibria of aqueous two-phase system containing polyethylene glycol di-methyl ether and ammonium sulfate and application of this system in separation of lactic acid. Fluid Phase Equilib. 459, 85–93 (2018)
Gómez, E., Calvar, N., Domínguez, Á., Macedo, E.A.: Thermal behavior and heat capacities of pyrrolidinium-based ionic liquids by DSC. Fluid Phase Equilib. 470, 51–59 (2018)
González, E.J., Díaz, I., Gonzalez-Miquel, M., Rodríguez, M., Sueiras, A.: On the behavior of imidazolium versus pyrrolidinium ionic liquids as extractants of phenolic compounds from water: experimental and computational analysis. Sep. Purif. Technol. 201, 214–222 (2018)
Verdía, P., González, E.J., Moreno, D., Palomar, J., Tojo, E.: Deepening of the role of cation substituents on the extractive ability of pyridinium ionic liquids of N-compounds from fuels. ACS Sustain. Chem. Eng. 5(2), 2015–2025 (2017)
Dong, Q., Muzny, C.D., Kazakov, A., Diky, V., Magee, J.W., Widegren, J.A., Chirico, R.D., Marsh, K.N., Frenkel, M.: ILThermo: a free-access web database for thermodynamic properties of ionic liquids. J. Chem. Eng. Data 52(4), 1151–1159 (2007)
Enayati, M., Mokhtarani, B., Sharifi, A., Anvari, S., Mirzaei, M.: Liquid–liquid extraction of toluene from alkane with pyridinium based ionic liquid ([BPy][NO3] and [HPy][NO3]) at 298.15 K and atmospheric pressure. J. Chem. Thermodyn. 102, 316–321 (2016)
Zhang, L., Lu, X., Ye, D., Guo, Y., Fang, W.: Density and viscosity for binary mixtures of the ionic liquid 2, 2-diethyl-1,1,3, 3-tetramethylguanidinium ethyl sulfate with water, methanol, or ethanol. J. Chem. Eng. Data 61(3), 1023–1031 (2016)
Gong, Y., Shen, C., Lu, Y., Meng, H., Li, C.: Viscosity and density measurements for six binary mixtures of water (methanol or ethanol) with an ionic liquid ([BMIM][DMP] or [EMIM][DMP]) at atmospheric pressure in the temperature range of (293.15 to 333.15) K. J. Chem. Eng. Data 57(1), 33–39 (2012)
Galvão, A.C., Robazza, W.S., Bianchi, A.D., Matiello, J.A., Paludo, A.R., Thomas, R.: Solubility and thermodynamics of vitamin C in binary liquid mixtures involving water, methanol, ethanol and isopropanol at different temperatures. J. Chem. Thermodyn. 121, 8–16 (2018)
Kestin, J., Sengers, J., Kamgar-Parsi, B., Sengers, J.L.: Thermophysical properties of fluid D2O. J. Phys. Chem. Ref. Data 13(2), 601–609 (1984)
Lin, C.-P., Lai, G.-H., Tu, C.-H.: Liquid–liquid equilibria, density, refractive index, and solubility for mixtures of water + methanol + heptane + methylbenzene or + dimethyl carbonate at T = 298.15 K. J. Chem. Eng. Data 58(11), 3265–3274 (2013)
Marsh, K.N.: Role of reference materials for the realization of physicochemical properties. Past, present, and future. Pure Appl. Chem. 72(10), 1809–1818 (2000)
Urréjola, S., Sánchez, A., Hervello, M.N.F.: Refractive indices of lithium, magnesium, and copper(II) sulfates in ethanol−water solutions. J. Phys. Chem. Ref. Data 55(1), 482–487 (2010)
Nikam, P.S., Shirsat, L.N., Hasan, M.: Density and viscosity studies of binary mixtures of acetonitrile with methanol, ethanol, propan-1-ol, propan-2-ol, butan-1-ol, 2-methylpropan-1-ol, and 2-methylpropan-2-ol at (298.15, 303.15, 308.15, and 313.15) K. J. Chem. Eng. Data 43(5), 732–737 (1998)
Henni, A., Hromek, J.J., Tontiwachwuthikul, P., Chakma, A.: Volumetric properties and viscosities for aqueous AMP solutions from 25 °C to 70 °C. J. Chem. Eng. Data 48(3), 551–556 (2003)
Mutalik, V., Manjeshwar, L.S., Sairam, M., Aminabhavi, T.M.: Excess molar volumes, deviations in viscosity and refractive index of the binary mixtures of mesitylene with ethanol, propan-1-ol, propan-2-ol, butan-1-ol, pentan-1-ol, and 3-methylbutan-1-ol at 298.15, 303.15, and 308.15 K. J. Mol. Liq. 129(3), 147–154 (2006)
Papanastasiou, G.E., Papoutsis, A.D., Kokkinidis, G.I.: Physical behavior of some reaction media. Density, viscosity, dielectric constant, and refractive index changes of ethanol–dioxane mixtures at several temperatures. J. Chem. Eng. Data 32(3), 377–381 (1987)
Shekaari, H., Zafarani-Moattar, M.T., Mirheydari, S.N.: Density, viscosity, speed of sound, and refractive index of a ternary solution of aspirin, 1-butyl-3-methylimidazolium bromide, and acetonitrile at different temperatures T = (288.15 to 318.15) K. J. Chem. Eng. Data 60(6), 1572–1583 (2015)
Moumouzias, G., Panopoulos, D.K., Ritzoulis, G.: Excess properties of the binary liquid system propylene carbonate + acetonitrile. J. Chem. Eng. Data 36(1), 20–23 (1991)
Iloukhani, H., Almasi, M.: Densities, viscosities, excess molar volumes, and refractive indices of acetonitrile and 2-alkanols binary mixtures at different temperatures: experimental results and application of the Prigogine–Flory–Patterson theory. Thermochim. Acta 495(1–2), 139–148 (2009)
Aminabhavi, T.M., Gopalakrishna, B.: Density, viscosity, refractive index, and speed of sound in aqueous mixtures of N, N-dimethylformamide, dimethyl sulfoxide, N, N-dimethylacetamide, acetonitrile, ethylene glycol, diethylene glycol, 1,4-dioxane, tetrahydrofuran, 2-methoxyethanol, and 2-ethoxyethanol at 298.15 K. J. Chem. Eng. Data 40(4), 856–861 (1995)
Zafarani-Moattar, M.T., Shekaari, H.: Volumetric and compressibility behaviour of ionic liquid, 1-n-butyl-3-methylimidazolium hexafluorophosphate and tetrabutylammonium hexafluorophosphate in organic solvents at T = 298.15 K. J. Chem. Thermodyn. 38(5), 624–633 (2006)
Redlich, O., Meyer, D.M.: The molal volumes of electrolytes. Chem. Rev. 64(3), 221–227 (1964)
Rogers, P., Pitzer, K.S.: Volumetric properties of aqueous sodium chloride solutions. J. Phys. Chem. Ref. Data 11(1), 15–81 (1982)
Shekaari, H., Zafarani-Moattar, M.T.: Volumetric properties of the ionic liquid, 1-butyl-3-methylimidazolium tetrafluoroborate, in organic solvents at T = 298.15 K. Int. J. Thermophys. 29(2), 534–545 (2008)
Jones, G., Dole, M.: The viscosity of aqueous solutions of strong electrolytes with special reference to barium chloride. J. Am. Chem. Soc. 51(10), 2950–2964 (1929)
Sah, R.S., Sinha, B., Roy, M.N.: Study of the solution properties of ternary mixtures of 1, 3-dioxolane (1), diethyl ether (2), and n-amyl alcohol (3) and the corresponding binary mixtures by density, viscosity, refractivity, and ultrasonic speed measurements at 298.15 K. J. Chem. Eng. Data 55(10), 4536–4540 (2010)
Ranjbar, S., Fakhri, K., Ghasemi, J.B.: Densities and viscosities of (1-propanol + 1, 2-dichloroethane), (1-propanol + benzaldehyde),(benzaldehyde + 1, 2-dichloroethane), and (1-propanol + 1, 2-dichloroethane + benzaldehyde) mixtures from T = 288.15 K to 313.15 K. J. Chem. Eng. Data 54(12), 3284–3290 (2009)
Mohammadi, M.D., Hamzehloo, M.: Densities, viscosities, and refractive indices of binary and ternary mixtures of methanol, acetone, and chloroform at temperatures from (298.15–318.15) K and ambient pressure. Fluid Phase Equilib. 483, 14–30 (2019)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Doust Mohammadi, M., Hamzehloo, M. & Abdullah, H.Y. Experimental Measurement of Physical, Transport, and Optical Properties of Binary Mixtures of N-Hexyl Pyridinium Nitrate [HPy][NO3] Ionic Liquid with Water, Ethanol, and Acetonitrile at 298.15 K and 101 kPa. J Solution Chem 50, 576–590 (2021). https://doi.org/10.1007/s10953-021-01078-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-021-01078-3