Abstract
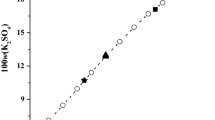
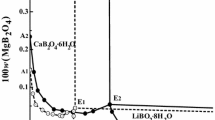
Solid–liquid phase equilibria for the two aqueous systems (LiBO2 + NaBO2 + H2O) and (LiBO2 + KBO2 + H2O) at T = 288.15 K and p = 0.1 MPa were determined using the isothermal dissolution equilibrium method. The experimental results show that the phase diagrams consist of one two-salt co-saturated invariant point, two univariant solubility isotherms, and three crystallization fields. The two systems belong to simple co-saturated type, and neither double salt nor solid solution were found. The densities change regularly as the sodium metaborate (potassium metaborate) concentration increases in solution, and reach their maximum values at the invariant point. Based on the Pitzer and its extended Harvie–Møller–Weare (HMW) model, the solubilities for the ternary systems at 288.15 K were represented, and the calculated results agree well with the experimental values.







Similar content being viewed by others
References
Wang, P.M., Kosinski, J.J., Lencka, M.M., Anderko, A., Springer, R.D.: Thermodynamic modeling of boric acid and selected metal borate systems. Pure Appl. Chem. 85, 2117–2144 (2013)
Li, J., Gao, S.Y., Xia, S.P., Li, B., Hu, R.Z.: Thermochemistry of hydrated magnesium borates. J. Chem. Thermodyn. 29, 491–497 (1997)
Wang, S.Q., Du, X.M., Jing, Y., Guo, Y.F., Deng, T.L.: Solid–liquid phase equilibrium in the ternary systems (Li2B4O7 + MgB4O7 + H2O) and (Na2B4O7 + MgB4O7 + H2O) at 298.15 K. J. Chem. Eng. Data 62, 253–258 (2017)
Gao, D.L., Guo, Y.F., Yu, X.P., Wang, S.Q., Deng, T.L.: Solubilities, densities, and refractive indices in the salt–water ternary system (LiCl + LiBO2 + H2O) at T = 288.15 K and 298.15 K and p = 0.1 MPa. J. Chem. Eng. Data 60, 2594–2599 (2015)
Wang, S.Q., Guo, Y.F., Liu, W.J., Deng, T.L.: Phase equilibria in the aqueous ternary system (LiBO2 + CaB2O4 + H2O) at 288.15 and 298.15 K. J. Solution Chem. 44, 1545–1554 (2015)
Cui, W.J., Hou, H.F., Hu, J.Y., Guo, Y.F., Deng, T.L.: Phase equilibria and phase diagrams for the aqueous ternary system containing sodium, chloride, and metaborate ions at 288.15 and 308.15 K and 0.1 MPa. J. Chem. 2019, 983051 (2019)
Chen, S.Q., Wang, M.X., Hu, J.Y., Guo, Y.F., Deng, T.L.: Phase equilibria in the aqueous ternary systems (NaCl + NaBO2 + H2O) and (Na2SO4 + NaBO2 + H2O) at 298.15 K and 0.1 MPa. J. Chem. Eng. Data 63, 4662–4668 (2018)
Vilarinho-Francoa, T., Teyssiera, A., Tenub, R., Pécautc, J., Delmasa, J., Heitzmanna, M., Caprona, P., Couniouxb, J.J., Goutaudier, C.: Solid–liquid equilibria in the ternary system NaBO2–NaOH–H2O thermal behavior of double salts. Fluid Phase Equilib. 360, 212–221 (2013)
Churikov, A.V., Zapsis, K.V., Khramkov, V.V., Churikov, M.A., Gamayunova, I.M.: Temperature-induced transformation of the phase diagrams of ternary systems NaBO2 + NaOH + H2O and KBO2 + KOH + H2O. J. Chem. Eng. Data 56, 383–389 (2011)
Wang, S.Q., Yang, J., Shi, C.C., Zhao, D., Guo, Y.F., Deng, T.L.: Solubilities, densities, and refractive indices in the ternary systems (LiBO2 + NaBO2 + H2O) and (LiBO2 + KBO2 + H2O) at 298.15 K and 0.1 MPa. J. Chem. Eng. Data 64, 3122–3127 (2019)
Guo, Y.F., Li, L., Cao, L.N., Yu, X.P., Wang, S.Q., Deng, T.L.: Solubilities, densities and refractive indices in the aqueous quaternary system of lithium sulfate, lithium metaborate, and lithium carbonate at 288.15, 298.15, 308.15 K and 0.1 MPa. J. Chem. Eng. Data 62, 508–515 (2017)
Cao, L.N., Li, L., Zhang, N., Guo, Y.F., Deng, T.L.: Phase equilibria of quaternary system LiCl–LiBO2–Li2SO4–H2O at 298.15 K. Ciesc. J. 67, 1117–1122 (2016)
Zhu, L.X., Gao, S.Y., Wang, B., Xia, S.P.: Study on thermochemistry of hydrated potassium monoborate. Chin. J. Inorg. Chem. 19, 333–336 (2013)
Wang, S.Q., Guo, Y.F., Li, D.C., Zhao, F.M., Deng, T.L.: Solid–liquid phase equilibria in the ternary systems (LiCl + MgCl2 + H2O) and (Li2SO4 + MgSO4 + H2O) at 288.15 K. J. Chem. Eng. Data 60, 821–827 (2015)
Wang, S.Q., Guo, Y.F., Li, D.C., Tang, P., Deng, T.L.: Experimental determination and modeling of the solubility phase diagram of the ternary system (Li2SO4 + K2SO4 + H2O) at 288.15 K. Thermochim. Acta 601, 75–81 (2015)
Song, P.S.: Studies on the application of the wet residues method in phase equilibrium for the salt–water systems. J. Salt Lake Res. 1, 4–46 (1991)
Nies, N.P., Hulbert, R.W.: Solubility isotherms in the system sodium oxide–boric oxide–water. Revised solubility–temperature curves of boric acid, borax, sodium pentaborate, and sodium metaborate. J. Chem. Eng. Data 12, 303–313 (1967)
Yang, Q., Li, J., Li, H.X., Zhang, F.X.: Study on the isothermal solubility for the ternary system Na2B4O7−NaHCO3−H2O and Na2CO3−NaBO2−H2O. Chin. J. Salt Lake Res. 9, 24–29 (2001)
Krol, O., Andrieux, J., Counioux, J.J., Tenu, R., Goutaudier, C.: Solubility and related equilibria in the KBO2–H2O and KBO2–H2O–KOH systems. In JEEP—35th Conference Phase Equilibria 2009, 00023 (2009)
Reburn, W.T., Gale, W.A.: The system lithium oxide–boric oxide–water. J. Phys. Chem. 59, 19–24 (1955)
Laversenne, L., Goutaudier, C., Chiriac, R., Sigala, C., Bonnetot, B.: Hydrogen storage in borohydrides: Comparison of hydrolysis conditions of LiBH4, NaBH4 and KBH4. J. Therm. Anal. Calorim. 94, 785–790 (2008)
Pitzer, K.S.: Thermodynamics, 3rd edn. McGraw-Hill Inc., New York (1995)
Pitzer, K.S.: Thermodynamics of electrolytes I: theoretical basis and general equations. J. Phys. Chem. 77, 268–277 (1973)
Harvie, C.E., Weare, J.H.: The prediction of mineral solubilities in natural waters: The Na–K–Mg–Ca–Cl–SO4–H2O system from zero to high concentration at 25 °C. Geochim. Cosmochim. Acta 44, 981–997 (1980)
Harvie, C.E., Eugster, H.P., Weare, J.H.: Mineral equilibria in the six-component seawater system Na–K–Mg–Ca–SO4–Cl–H2O at 25 °C. II: compositions of the saturated solutions. Geochim. Cosmochim. Acta 46, 1603–1618 (1982)
Harvie, C.E., Møller, N., Weare, J.H.: The prediction of mineral solubilities in natural waters: The Na–K–Mg–Ca–H–Cl–SO4–OH–HCO3–CO3–CO2–H2O system to high ionic strenghs at 25 °C. Geochim. Cosmochim. Acta 48, 723–751 (1984)
Sirnonson, J.M., Roy, R.N., Roy, L.N., Johnson, D.A.: The thermodynamics of aqueous borate solutions I. Mixtures of boric acid with sodium or potassium borate and chloride. J. Solution. Chem. 16, 792–803 (1987)
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (U1707602, U1507109, U1607123, 21773170, and 21106103), the Natural Science Foundation of Tianjin (17JCYBJC19500), and the Yangtze Scholars and Innovative Research Team in University of Ministry of Education of China (IRT-17R81).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, S., Shi, C., Yang, J. et al. Solid–Liquid Phase Equilibria in the Ternary Systems (LiBO2 + NaBO2 + H2O) and (LiBO2 + KBO2 + H2O) at 288.15 K and 0.1 MPa. J Solution Chem 49, 353–364 (2020). https://doi.org/10.1007/s10953-020-00962-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-020-00962-8