Abstract
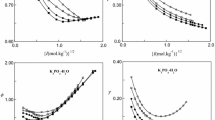
Water activities and stoichiometric osmotic coefficients for the systems MgB4O7+H2O and MgSO4+MgB4O7+H2O have been measured at 298.15 K by the isopiestic method using a improved isopiestic device; the saturated solution molalities of MgB4O7 are very low for these systems. These measurements extended from the near saturated molalities to supersaturation for the MgB4O7 binary solutions and from total molalities m T of 0.1787 to 2.2374 mol⋅kg−1 with seven MgB4O7 molality fractions from 0.005 to 0.095 for the ternary solutions, respectively. The water activities of MgSO4 binary solutions (Y B = 0.0) were obtained by extrapolation of the present experimental results and were in agreement with the data from Rard and Miller (J. Chem. Eng. Data 26:33–38, 1981). The experimental stoichiometric osmotic coefficients are represented using Pitzer’s ion-interaction model with a modified value of α B2=7.59 kg1/2⋅mol−1/2 in the term, \(B_{\mathrm{Mg,B}_{4}\mathrm{O}_{7}}^{\phi}\) . Two sets of ion-interaction model parameters are presented for MgSO4+MgB4O7+H2O. The mixing parameters of the first set were evaluated using the presently calculated MgB4O7 single-salt parameters obtained from its binary-solution data. All the parameters relative to borate in the second set were estimated simultaneously from all the measured stoichiometric osmotic coefficient data for binary and ternary solutions in the present work, and were obtained with standard deviations of 0.0022 for MgB4O7 single salt-parameters and 0.0063 for the mixing parameters. The MgSO4 single-salt parameters reported by Rard and Miller (J. Chem. Eng. Data 26:33–38, 1981) were used for the evaluation of both sets of the ion-interaction parameters. The stoichiometric mean activity coefficients of the solutes for the systems are primarily calculated using Pitzer’s standard equations for the activity coefficient with the same values of parameters and the exponential coefficients of α 1, α 2 and α B2 for the osmotic coefficient model. The effects of the ionic interactions on the thermodynamic properties for the studied systems are discussed.
Similar content being viewed by others
References
Rard, J.A., Platford, R.F.: Experimental methods: isopiestic. In: Pitzer, K.S. (ed.) Activity Coefficients in Electrolyte Solutions, vol. 5, 2nd edn, pp. 209–273. CRC Press, Boca Raton (1991)
Rard, R.A., Miller, R.G.: Isopiestic determination of the osmotic coefficients of aqueous Na2SO4, MgSO4, and Na2SO4−MgSO4 at 25 °C. J. Chem. Eng. Data 26, 33–38 (1981)
Rard, J.A.: Aqueous solubilities of praseodymium. europium, and lutetium sulfates. J. Solution Chem. 17, 499–517 (1988)
Rard, J.A.: Isopiestic determination of the osmotic and activity coefficients of {(1−y)H2SO4+yNa2SO4}(aq) at 298.15 K. I. Results for y=0.5(NaHSO4) and y=0.55595, 0.70189, and 0.84920. J. Chem. Thermodyn. 21, 539–560 (1989)
Rard, J.A.: Isopiestic determination of the osmotic and activity coefficients of aqueous Lu2(SO4)3 at 25 °C. J. Solution Chem. 19, 525–541 (1990)
Rard, J.A.: Isopiestic determination of the osmotic and activity coefficients of {(1−y)H2SO4+yNa2SO4}(aq) at the temperature 298.15 K II. Results for y=(0.12471, 0.24962, and 0.37439). J. Chem. Thermodyn. 24, 45–66 (1992)
Hovey, J.K., Pitzer, K.S., Rard, R.A.: Thermodynamics of Na2SO4(aq) at temperature T from 273 K to 373 K and of {(1−y)H2SO4+yNa2SO4}(aq) at T=298.15 K. J. Chem. Thermodyn. 25, 173–192 (1993)
Rard, J.A.: Isopiestic determination of the osmotic and activity coefficients of {zH2SO4+(1−z)MgSO4}(aq) at the temperature T=298.15 K. I. Results for z=(0.85811, 0.71539, and 0.57353). J. Chem. Thermodyn. 29, 533–555 (1997)
Archer, D.G., Rard, J.A.: Isopiestic investigation of the osmotic and activity coefficients of aqueous MgSO4 and the solubility of the MgSO4+H2O system to 440 K. J. Chem. Eng. Data 43, 791–806 (1998)
Palmer, D.A., Rard, J.A., Clegg, S.L.: Isopiestic determination of the osmotic and activity coefficients of Rb2SO4(aq) and Cs2SO4(aq) at T=(298.15 and 323.15) K, and representation with an extended ion-interaction (Pitzer) model. J. Chem. Thermodyn. 34, 63–102 (2002)
Rard, J.A., Clegg, S.L.: Critical evaluation of the thermodynamic properties of aqueous calcium chloride. 1. Osmotic and activity coefficients of 0–10.77 mol⋅kg−1 aqueous calcium chloride solutions at 298.15 K and correlation with extended Pitzer ion-interaction models. J. Chem. Eng. Data 42, 819–849 (1997)
Pitzer, K.S., Wang, P.-M., Rard, J.A., Clegg, S.L.: Thermodynamics of electrolytes. 13. Ionic strength dependence of higher-order terms: Equations for CaCl2 and MgCl2. J. Solution Chem. 28, 265–289 (1999)
Rard, J.A., Miller, D.G.: Isopiestic determination of the osmotic and coefficients of aqueous MgCl2 solutions at 25 °C. J. Chem. Eng. Data 26, 38–43 (1981)
Rard, J.A., Wijesinghe, A.M., Wolery, T.J.: Review of the thermodynamic properties of Mg(NO3)2(aq) and their representation with the standard and extended ion-interaction (Pitzer) models at 298.15 K. J. Chem. Eng. Data 49, 1127–1140 (2004)
Clegg, S.L., Rard, J.A., Miller, D.G.: Isopiestic determination of the osmotic and activity coefficients of NaCl+SrCl2+H2O at 298.15 K and representation with an extended ion-interaction model. J. Chem. Eng. Data 50, 1162–1170 (2005)
Archer, D.G., Wood, R.H.: Chemical equilibrium model applied to aqueous magnesium sulfate solutions. J. Solution Chem. 14, 757–779 (1985)
Holmes, H.F., Mesmer, R.E.: Isopiestic studies of aqueous solutions at elevated temperatures. VII. MgSO4 and NiSO4. J. Chem. Thermodyn. 15, 709–719 (1983)
Pillay, V., Gartner, R.S., Himawan, C., Seckler, M.M., Lewis, A.E., Witkamp, G.-J.: MgSO4+H2O system at eutectic conditions and thermodynamic solubility products of MgSO4⋅12H2O (s) and MgSO4⋅7H2O (s). J. Chem. Eng. Data 50, 551–555 (2005)
Zhang, Z., Yao, Y., Song, P.-S., Chen, J.-Q.: Isopiestic determination of the osmotic and activity coefficients of aqueous mixtures of Li2SO4 and MgSO4. Acta Phys.-Chim. Sinica 9, 366–373 (1993)
Clegg, S.L., Whitfield, M.: Activity coefficients in natural waters. In: Pitzer, K.S. (ed.) Activity Coefficients in Electrolyte Solutions, 2nd edn. CRC Press, Boca Raton (1991)
Felmy, A.R., Weare, J.H.: The prediction of borate mineral equilibria in natural waters: Application to Searles Lake, California. Geochim. Cosmochim. Acta 50, 2771–2783 (1986)
Ingri, N., Largerström, G., Frydman, M., Sillen, L.G.: Equilibrium studies of polyanions. II. Polyborates in NaClO4 medium. Acta Chem. Scand. 11, 1034–1058 (1957)
Ingri, N.: Equilibrium studies of polyanions. 8. On the first equilibrium steps in the hydrolysis of boric acid, a comparison between equilibria in 0.1 M and 3.0 M NaClO4. Acta Chem. Scand. 16, 439–448 (1962)
Ingri, N.: Equilibrium studies of polyanions. 10. On the first equilibrium steps in the acidification of B(OH) −4 , on the application of the self-medium method. Acta Chem. Scand. 17, 573–580 (1963)
Mesmer, R.E., Baes, C.F., Sweeton, F.H.: Acidity measurements at elevated temperatures. VI. Boric acid equilibria. Inorg. Chem. 11, 537–543 (1972)
Maya, L.: Identification of polyborate and fluoropolyborate ions in solution by Raman spectroscopy. Inorg. Chem. 15, 2179–2184 (1976)
Maeda, M., Hirao, T., Kotaka, M., Kakihana, H.: Raman spectra of polyborate ions in aqueous solution. J. Inorg. Nucl. Chem. 41, 1217–1220 (1979)
Bassett, R.L.: A critical evaluation of the thermodynamic data for boron ions. Ion pairs, complexes, and polyanions in aqueous solution at 298.15 K and 1 bar. Geochim. Cosmochim. Acta 44, 1151–1160 (1980)
Gao, S.-Y., Wang, J.-Z., Xia, S.-P.: Chemistry of borate in Salt Lake Brine VIII. The form of borate existing in concentrated brine and its expression. Oceanol. Limnol. Sinica 20, 429–437 (1989)
Jia, Y.-Z.: Vibrational spectroscopy of aqueous solutions and physical chemistry of borates. Ph.D. Thesis, Lanzhou University (2000)
Song, P.-S., Du, X.-H., Sun, B.: Study on the ternary system MgB4O7−MgSO4−H2O at 25 °C. Kexue Tongbao 33, 1971–1973 (1988)
Harvie, C.E., Møller, N., Weare, J.H.: The prediction of mineral solubilities in natural waters: the Na–K–Mg–Ca–H–Cl–SO4–OH–HCO3–CO3–CO2–H2O system to high ionic strengths at 25 °C. Geochim. Cosmochim. Acta 48, 723–751 (1984)
Platford, R.F.: Osmotic and activity coefficients of some simple borates in aqueous solution at 25 °C. Can. J. Chem. 47, 2271–2273 (1969)
Zhang, A.-Y., Yao, Y., Li, L.-J., Song, P.-S.: Isopiestic determination of the osmotic coefficients and Pitzer model representation for Li2B4O7(aq) at T=298.15 K. J. Chem. Thermodyn. 37, 101–109 (2005)
Yang, J.-M., Yao, Y., Zhang, A.-Y., Song, P.-S.: Isopiestic studies on thermodynamic properties for LiCl−Li2B4O7−H2O system at 298.15 K. Chem. J. Chin. Univ. 27, 735–738 (2006)
Yuan, W.-P., Yao, Y., Song, P.-S.: Isopiestic studies on the thermodynamic properties for Li2B4O7−Li2SO4−H2O system at 298.15 K. J. Salt Lake Res. 13, 29–34 (2005)
Zhang, A.-Y., Yao, Y., Yang, J.-M., Song, P.-S.: Isopiestic studies of thermodynamic properties and representation with ion-interaction model for Li2B4O7−MgCl2(B)−H2O system at 298.15 K. Acta Chim. Sinica 62, 1089–1094 (2004)
Zhang, A.-Y., Yao, Y., Yang, J.-M.: Studies on thermodynamic properties by isopiestic and EMF method and ion-interaction model for Li2B4O7−MgCl2−H2O at T=298.15 K. The 18th IUPAC International Conference on Chemical Thermodynamics and The 12th National Conference on Chemical Thermodynamics and Thermal Analysis, Abstract 73 (2004)
Tian, H.-B., Yao, Y., Song, P.-S.: Studies of activity coefficients of LiCl and association equilibrium for LiCl−Li2B4O7−H2O. Chem. Res. Appl. 12, 403–408 (2000) (CA: Huaxue Yanjiu Yu Yingyong (Ch))
Yang, J.-Z., Sun, B., Song, P.S.: Thermodynamics of ionic association 1. The standard association constant of the ion pair Li+B(OH) −4 . Thermochim. Acta 352–353, 69–74 (2000)
Yin, G.-Y., Yao, Y., Jiao, B.-J., Chen, S.-P., Gao, S.-L.: Enthalpies of dilution of aqueous Li2B4O7 solutions at 298.15 K and application of ion-interaction model. Thermochim. Acta 435, 125–128 (2005)
Zhang, T.-H., Yin, G.-Y., Tan, Z.-Ch., Yao, Y., Sun, L.-X.: Heat capacities and thermodynamic properties of a H2O+Li2B4O7 solution in the temperature range from 80 to 356 K. J. Solution Chem. 35, 1347–1355 (2006)
Rard, J.A.: Solubility determinations by the isopiestic method and application to lanthanide nitrates at 25 °C. J. Solution Chem. 14, 457–471 (1985)
Pitzer, K.S., Mayorga, G.: Thermodynamics of electrolytes. III. Activity and osmotic coefficients for 2-2 electrolytes. J. Solution Chem. 3, 539–546 (1974)
Pitzer, K.S.: Ion interaction approach: theory and data correlation. In: Pitzer, K.S. (ed.) Activity Coefficients in Electrolyte Solutions, vol. 3, 2nd edn, pp. 75–153. CRC Press, Boca Raton (1991)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yin, S.T., Yao, Y., Li, B. et al. Isopiestic Studies of Aqueous MgB4O7 and MgSO4 + MgB4O7 at 298.15 K and Representation with Pitzer’s Ion-Interaction Model. J Solution Chem 36, 1745–1761 (2007). https://doi.org/10.1007/s10953-007-9211-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-007-9211-9