Abstract
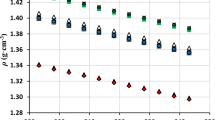
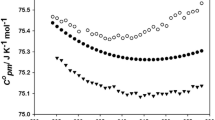
In this work, thermophysical properties of n-ethylpyridinium bis(trifluoromethylsulfonyl)imide have been studied at atmospheric pressure in the temperature range 288.15–338.15 K. Density, speed of sound, refractive index, surface tension, isobaric molar heat capacity, electrical conductivity and kinematic viscosity have been measured; from these data the isobaric expansibility, isentropic compressibility, molar refraction, entropy and enthalpy of surface formation per unit of surface area, and dynamic viscosity have been calculated. Moreover, we have characterized the thermal behavior of the compound. Results have been analyzed paying special attention to the structural and energetic factors. The magnitude and directionality of the cation–anion interactions have been studied using ab initio quantum calculations, which allow a better understanding of the physicochemical behavior of the ionic liquid. Finally, density values and radial distribution functions were also estimated ab initio from classical molecular dynamics simulations, providing acceptable density predictions.









Similar content being viewed by others
References
Wasserscheid, P., Welton, T.: Ionic Liquids in Synthesis. Wiley, Weinheim (2003)
Ishikawa, M., Sugimoto, T., Kikuta, M., Ishiko, E., Kono, M.: Pure ionic liquid electrolytes compatible with a graphitized carbon negative electrode in rechargeable lithium-ion batteries. J. Power Sources 162, 658–662 (2006)
Gan, Q., Rooney, D., Xue, M., Thompson, G., Zou, Y.: An experimental study of gas transport and separation properties of ionic liquids supported on nanofiltration membranes. J. Membr. Sci. 280, 948–956 (2006)
Torimoto, T., Tsuda, T., Okazaki, K., Kuwabata, S.: New frontiers in materials science opened by ionic liquids. Adv. Mater. 22, 1196–1221 (2010)
Bermudez, M.-D., Jimenez, A.-E., Sanes, J., Carrion, F.-J.: Ionic liquids as advanced lubricant fluids. Molecules 14, 2888–2908 (2009)
Pereiro, A.B., Araujo, J.M.M., Esperanca, J.M.S.S., Marrucho, I.M., Rebelo, L.P.N.: Ionic liquids in separations of azeotropic systems—a review. J. Chem. Thermodyn. 46, 2–28 (2012)
Moniruzzaman, M., Nakashima, K., Kamiya, N., Goto, M.: Recent advances of enzymatic reactions in ionic liquids. Biochem. Eng. J. 48, 295–314 (2010)
Kato, R., Gmehling, J.: Activity coefficients at infinite dilution of various solutes in the ionic liquids [MMIM]+[CH3SO4]−, [MMIM]+[CH3OC2H4SO4]−, [MMIM]+[(CH3)2PO4]−, [C5H5NC2H5]+[(CF3SO2)(2)N]− and [C5H5NH]+[C2H5OC2H4OSO3]−. Fluid Phase Equilib. 226, 37–44 (2004)
Liu, Q.-S., Yang, M., Yan, P.-F., Liu, X.-M., Tan, Z.-C., Welz-Biermann, U.: Density and surface tension of ionic liquids [C(n)py][NTf2] (n = 2, 4, 5). J. Chem. Eng. Data 55, 4928–4930 (2010)
Zhang, Q.-G., Sun, S–.S., Pitula, S., Liu, Q.-S., Welz-Biermann, U., Zhang, J.-J.: Electrical conductivity of solutions of ionic liquids with methanol, ethanol, acetonitrile, and propylene carbonate. J. Chem. Eng. Data 56, 4659–4664 (2011)
Bittner, B., Wrobel, R.J., Milchert, E.: Physical properties of pyridinium ionic liquids. J. Chem. Thermodyn. 55, 159–165 (2012)
Gutmann, T., Sellin, M., Breitzke, H., Stark, A., Buntkowsky, G.: Para-hydrogen induced polarization in homogeneous phase—an example of how ionic liquids affect homogenization and thus activation of catalysts. Phys. Chem. Chem. Phys. 11, 9170–9175 (2009)
Garcia-Mardones, M., Bandres, I., Carmen Lopez, M., Gascon, I., Lafuente, C.: Experimental and theoretical study of two pyridinium-based ionic liquids. J. Solut. Chem. 41, 1836–1852 (2012)
Mokhtarani, B., Sharifi, A., Mortaheb, H.R., Mirzaei, M., Mafi, M., Sadeghian, F.: Density and viscosity of pyridinium-based ionic liquids and their binary mixtures with water at several temperatures. J. Chem. Thermodyn. 41, 323–329 (2009)
Gu, Z.Y., Brennecke, J.F.: Volume expansivities and isothermal compressibilities of imidazolium and pyridinium-based ionic liquids. J. Chem. Eng. Data 47, 339–345 (2002)
Matkowska, D., Goldon, A., Hofman, T.: Densities, excess volumes, isobaric expansivities, and isothermal compressibilities of the 1-ethyl-3-methylimidazolium ethylsulfate plus ethanol system at temperatures (283.15 to 343.15 K) and pressures from 0.1 to 35 MPa. J. Chem. Eng. Data 55, 685–693 (2010)
Carvalho, P.J., Freire, M.G., Marrucho, I.M., Queimada, A.J., Coutinho, J.A.P.: Surface tensions for the 1-alkyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ionic liquids. J. Chem. Eng. Data 53, 1346–1350 (2008)
Crosthwaite, J.M., Muldoon, M.J., Dixon, J.K., Anderson, J.L., Brennecke, J.F.: Phase transition and decomposition temperatures, heat capacities and viscosities of pyridinium ionic liquids. J. Chem. Thermodyn. 37, 559–568 (2005)
Liu, H., Maginn, E., Visser, A.E., Bridges, N.J., Fox, E.B.: Thermal and transport properties of six ionic liquids: an experimental and molecular dynamics study. Ind. Eng. Chem. Res. 51, 7242–7254 (2012)
Ngo, H.L., LeCompte, K., Hargens, L., McEwen, A.B.: Thermal properties of imidazolium ionic liquids. Thermochim. Acta 357, 97–102 (2000)
Vogel, H.: Das Temperatur-abhängigkeitsgesetz der viskosität von flüssigkeiten (The temperature-independence law of viscosity of liquids). Phys. Zeit. 22, 645–646 (1921)
Tammann, G., Hesse, W.: Die Abhängigkeit der viscosität von der temperatur bei unterkühlten flüssigkeiten (The temperature dependence of the viscosity of supercooled fluids). Zeitschrift für anorganische und allgemeine Chemie 156, 245–257 (1926)
Fulcher, G.S.: Analysis of recent measurements of the viscosity of glasses. J. Am. Ceram. Soc. 8, 339–355 (1923)
Yu, Y.-H., Soriano, A.N., Li, M.-H.: Heat capacities and electrical conductivities of 1-n-butyl-3-methylimidazolium-based ionic liquids. Thermochim. Acta 482, 42–48 (2009)
Vila, J., Varela, L.M., Cabeza, O.: Cation and anion sizes influence in the temperature dependence of the electrical conductivity in nine imidazolium based ionic liquids. Electrochim. Acta 52, 7413–7417 (2007)
Gardas, R.L., Coutinho, J.A.P.: A group contribution method for viscosity estimation of ionic liquids. Fluid Phase Equilib. 266, 195–201 (2008)
Fredlake, C.P., Crosthwaite, J.M., Hert, D.G., Aki, S., Brennecke, J.F.: Thermophysical properties of imidazolium-based ionic liquids. J. Chem. Eng. Data 49, 954–964 (2004)
Seoane, R.G., Corderi, S., Gomez, E., Calvar, N., Gonzalez, E.J., Macedo, E.A., Dominguez, A.: Temperature dependence and structural influence on the thermophysical properties of eleven commercial ionic liquids. Ind. Eng. Chem. Res. 51, 2492–2504 (2012)
Izgorodina, E.I.: Towards large-scale, fully ab initio calculations of ionic liquids. Phys. Chem. Chem. Phys. 13, 4189–4207 (2011)
Rees, R.J., Lane, G.H., Hollenkamp, A.F., Best, A.S.: Predicting properties of new ionic liquids: density functional theory and experimental studies of tetra-alkylammonium salts of (thio)carboxylate anions, RCO2 −, RCOS− and RCS2 −. Phys. Chem. Chem. Phys. 13, 10729–10740 (2011)
Frisch, G.W.T.M.J., Pople, J.A., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Montgomery Jr., J.A., Vreven, T., Kudin, K.N., Burant, J.C., Millam, J.M., Iyengar, S.S., Tomasi, J., Barone, V., Mennucci, B., Cossi, M., Scalmani, G., Rega, N., Petersson, G.A., Nakatsuji, H., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Klene, M., Li, X., Knox, J.E., Hratchian, H.P., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Ayala, P.Y., Morokuma, K., Voth, G.A., Salvador, P., Dannenberg, J.J., Zakrzewski, V.G., Dapprich, S., Daniels, A.D., Strain, M.C., Farkas, O., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Ortiz, J.V., Cui, Q., Baboul, A.G., Clifford, S., Cioslowski, J., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Martin, R.L., Fox, D.J., Keith, T., Al-Laham, M.A., Peng, C.Y., Nanayakkara, A., Challacombe, M., Gill, P.M.W., Johnson, B., Chen, W., Wong, M.W., Gonzalez, C.: Gaussian. Gaussian Inc., Wallingford, CT (2004)
Xuan, X., Guo, M., Pei, Y., Zheng, Y.: Theoretical study on cation–anion interaction and vibrational spectra of 1-allyl-3-methylimidazolium-based ionic liquids. Spectrochim. Acta A 78, 1492–1499 (2011)
Tsuzuki, S., Tokuda, H., Hayamizu, K., Watanabe, M.: Magnitude and directionality of interaction in ion pairs of ionic liquids: relationship with ionic conductivity. J. Phys. Chem. B. 109, 16474–16481 (2005)
Hess, B., Kutzner, C., van der Spoel, D., Lindahl, E.: GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theory Comput. 4, 435–447 (2008)
Hockney, R., Goel, S., Eastwood, J.: Quiet high-resolution computer models of a plasma. J. Comput. Phys. 14, 148–158 (1974)
Bussi, G., Donadio, D., Parrinello, M.: Canonical sampling through velocity rescaling. J. Chem. Phys. 126, 014101 (2007)
Berendsen, H., Postma, J., Vangunsteren, W., Dinola, A., Haak, J.: Molecular-dynamics with coupling to an external bath. J. Chem. Phys. 81, 3684–3690 (1984)
Wang, J.M., Wolf, R.M., Caldwell, J.W., Kollman, P.A., Case, D.A.: Development and testing of a general amber force field. J. Comput. Chem. 25, 1157–1174 (2004)
Essmann, U., Perera, L., Berkowitz, M., Darden, T., Lee, H., Pedersen, L.: A smooth particle mesh Ewald method. J. Chem. Phys. 103, 8577–8593 (1995)
Acknowledgments
The authors gratefully acknowledge financial support from Diputación General de Aragón and Fondo Social Europeo “Construyendo Europa desde Aragón”.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Benito, J., García-Mardones, M., Pérez-Gregorio, V. et al. Physicochemical Study of n-Ethylpyridinium bis(trifluoromethylsulfonyl)imide Ionic Liquid. J Solution Chem 43, 696–710 (2014). https://doi.org/10.1007/s10953-014-0156-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-014-0156-5