Abstract
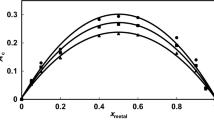
The main aim of this research is to study the complexation of molybdenum(VI) with methyliminodiacetic acid in NaClO4 aqueous solutions at pH = 6.00 and ionic strengths (0.1<I/mol⋅dm−3<1.0) at 25 °C by using potentiometric and UV spectrophotometric measurements in order to obtain thermodynamic stability constants at I=0 mol⋅dm−3. A comparison with previous literature data was made for the stability constants, though few data were available. The stability constants data have been analyzed and interpreted by using extended Debye-Hückel theory, specific ion interaction theory and parabolic model. Finally it might be concluded that parabolic model applies better for this complexation reaction.
Similar content being viewed by others
References
Bertini, I., Gray, H.B., Stiefel, E.I., Valentine, J.S.: Biological Inorganic Chemistry, Structure and Reactivity, 1st edn. University Science Books, Sausalito (2007)
Anderegg, G., Arnaud-Neu, F., Delgado, R., Felcman, J., Popov, K.: Critical evaluation of stability constants of metal complexes of complexones for biomedical and environmental applications. Pure Appl. Chem. 77, 1445–1495 (2005)
Majlesi, K., Rezaienejad, S.: Application of the parabolic model, specific ion interaction, and Debye-Hückel theories for the complexation of dioxovanadium(V) with ethylenediamine-N,N′-diacetic acid. J. Chem. Eng. Data 54, 1483–1492 (2009)
Majlesi, K., Rezaienejad, S.: Application of specific ion interaction theory and parabolic models for the molybdenum(VI) and tungsten(VI) complexes with NTA and IDA at different ionic strengths. Chin. Chem. Lett. 20, 759–762 (2009)
Majlesi, K., Zare, K., Rezaienejad, S., Nemati, F.: Comparison of the application of Debye-Hückel and specific ion interaction theories for the complexation of tungsten(VI) with ethylenediaminediacetic acid. Russ. J. Inorg. Chem. 54, 803–807 (2009)
Majlesi, K., Rezaienejad, S.: Complexation of dioxovanadium(V) with methyliminodiacetic acid in NaClO4 aqueous solutions at different ionic strengths by using an extended Debye-Hückel equation, specific ion interaction theory, and parabolic equations. J. Chem. Eng. Data 55, 882–888 (2010).
Majlesi, K., Momeni, N.: Complexation of molybdenum(VI) with ethylenediaminediacetic acid in different water + methanol solutions. J. Chem. Eng. Data 54, 2479–2482 (2009)
Zare, K., Lagrange, P., Lagrange, J.: Determination and comparison of stability constants of vanadium(V), molybdenum(VI) and tungsten(VI) aminocarboxylate complexes. J. Chem. Soc., Dalton Trans. 1372–1376 (1979)
Billo, J.E.: Excel for Chemists, 2nd edn. Wiley, New York (2001)
Kula, R.J.: Solution equilibria and structures of molybdenum(VI) chelates, N-methyliminodiacetic acid. Anal. Chem. 38, 1382–1388 (1966)
Daniele, P.G., Rigano, C., Sammartano, S.: Ionic strength dependence of formation constants. Alkali metal complexes of EDTA, NTA, diphosphate and tripolyphosphate in aqueous solution. Anal. Chem. 57, 2956–2960 (1985)
Daniele, P.G., Rigano, C., Sammartano, S., Zelano, V.: Ionic strength dependence of formation constants—XVIII. The hydrolysis of iron(III) in aqueous KNO3 solutions. Talanta 41, 1577–1582 (1994)
De Stefano, C., Gianguzza, A., Piazzese, D., Sammartano, S.: Polyacrylate protonation in various aqueous ionic media at different temperatures and ionic strengths. J. Chem. Eng. Data 45, 876–881 (2000)
De Robertis, A., De Stefano, C., Foti, C.: Medium effects on the protonation of carboxylic acids at different temperatures. J. Chem. Eng. Data 44, 262–270 (1999)
Wang, M., Zhang, Y., Muhammed, M.: Critical evaluation of thermodynamics of complex formation of metal ions in aqueous solutions. I. A description of evaluation methods. Hydrometallurgy 45, 21–36 (1997)
Crea, F., De Stefano, C., Milea, D., Sammartano, S.: Speciation of phytate ion in aqueous solution. Thermodynamic parameters for zinc(II) sequestration at different ionic strengths and temperatures. J. Solution Chem. 38, 115–134 (2009)
Crea, F., Foti, C., Sammartano, S.: Sequestering ability of polycarboxylic ligands towards dioxouranium(VI). Talanta 75, 775–785 (2008)
Crea, P., De Stefano, C., Kambarami, M., Millero, F.J., Sharma, V.K.: Effect of ionic strength and temperature on the protonation of oxidized glutathione. J. Solution Chem. 37, 1245–1259 (2008)
Sipos, P.: Application of the specific ion interaction theory (SIT) for the ionic products of aqueous electrolyte solutions of very high concentrations. J. Mol. Liq. 143, 13–16 (2008)
Bretti, C., Crea, F., De Stefano, C., Sammartano, S.: Solubility and activity coefficients of 2,2′-bipyridyl, 1,10-phenanthroline and 2,2′,6′,2″-terpyridine in NaCl(aq) at different ionic strengths and T=298.15 K. Fluid Phase Equilib. 272, 47–52 (2008)
Bretti, C., Crea, F., Giuffre, O., Sammartano, S.: The effect of different aqueous ionic media on the acid-base properties of some open chain polyamines. J. Solution Chem. 37, 183–201 (2008)
Bretti, C., De Stefano, C., Millero, F.J., Sammartano, S.: Modeling of protonation constants of linear aliphatic dicarboxylates containing-S-groups in aqueous chloride salt solutions, at different ionic strengths, using the SIT and Pitzer equations and empirical relationships. J. Solution Chem. 37, 763–784 (2008)
Berto, S., Daniele, P.G., Foti, C., Prenesti, E., Sammartano, S.: Interaction of oxovanadium(IV) with carboxylic ligands in aqueous solution: a thermodynamic and visible spectrophotometric study. J. Mol. Liq. 142, 57–63 (2008)
Bretti, C., Cigala, R.M., Crea, F., Foti, C., Sammartano, S.: Solubility and activity coefficients of acidic and basic non-electrolytes in aqueous salt solutions: 3. Solubility and activity coefficients of adipic and pimelic acids in NaCl(aq), (CH3)4NCl(aq) and (C2H5)4NI(aq) at different ionic strengths and at t=25 ○C. Fluid Phase Equilib. 263, 43–54 (2008)
Cigala, R.M., Crea, F., Sammartano, S.: Mixing effects on the protonation of polyacrylates in LiCl/KCl aqueous solutions at different ionic strengths, I=1 to 3.5 mol⋅L−1, at T=298.15 K. J. Mol. Liq. 143, 129–133 (2008)
Battaglia, G., Cigala, R.M., Crea, F., Sammartano, S.: Solubility and acid-base properties of ethylenediaminetetraacetic acid in aqueous NaCl solution at 0<I<6 mol⋅kg−1 and T=298.15 K. J. Chem. Eng. Data 53, 363–367 (2008)
Thakur, P., Mathur, J.N., Moore, R.C., Choppin, G.R.: Thermodynamics and dissociation constants of carboxylic acids at high ionic strength and temperature. Inorg. Chim. Acta 360, 3671–3680 (2007)
Crea, F., De Stefano, C., Foti, C., Sammartano, S.: SIT parameters for the dependence of (poly) carboxylate activity coefficients on ionic strength in (C2H4)4NIaq (0<I<1.2 mol⋅kg−1) and (CH3)4NClaq (0<I<3.9 mol⋅kg−1) in the temperature range 278 K <T<328 K and correlation with Pitzer parameters. J. Chem. Eng. Data 52, 2195–2203 (2007)
Crea, P., De Stefano, C., Milea, D., Porcino, N., Sammartano, S.: Speciation of phytate ion in aqueous solution. Protonation constants and copper(II) interactions in NaNO3 aq at different ionic strengths. Biophys. Chem. 128, 176–184 (2007)
Crea, F., De Stefano, C., Milea, D., Sammartano, S.: Dioxouranium(VI)-carboxylate complexes. Speciation of \(\mathrm{UO}_{2}^{2+}\)-1,2,3-propanetricarboxylate system in NaCl(aq) at different ionic strengths and at t=25 ○C. Ann. Chim. 97, 163–175 (2007)
Crea, F., De Robertis, A., De Stefano, C., Sammartano, S.: Dioxouranium(VI)-carboxylate complexes, a calorimetric and potentiometric investigation of interaction with oxalate at infinite dilution and in NaCl aqueous solution at I=1.0 mol⋅L−1 and T=25 ○C. Talanta 71, 948–963 (2007)
Grenthe, I., Wanner, H.: TDB-2 guidelines for the extrapolation to zero ionic strength. Minor revisions by Östhols, Erik. http://www.nea.fr/html/dbtdb/guidelines/tdb2.pdf. Version of 6th January (2000)
Ciavatta, L.: The specific interaction theory in evaluating ionic equilibria. Ann. Chim. 70, 551–567 (1980)
Scatchard, G.: Equilibrium in Solution: Surface and Colloid Chemistry. Harvard University Press, Cambridge (1976)
Brown, P.L., Curti, E., Gambrow, B.: Chemical Thermodynamics of Zirconium, vol. 8, p. 33. Elsevier, Amsterdam (2005)
Thoenen, T., Hummel, W.: Application of the Brønsted-Guggenheim-Scatchard specific ion interaction theory to the concentration dependence of complexation constants in NaCl solutions up to the saturation of halite. J. Conf. Abstr. 5, 997 (2000)
Mitting, D., Choppin, G.R.: In: Reed, D.T., Clark, S.B., Rao, L. (eds.) Actinide Speciation in High Ionic Strength Media. Kluwer Academic/Plenum, New York (1999)
Yamada, S., Nagase, J., Funahashi, S., Tanaka, M.: Thermodynamic studies on complexation of pervanadyl ion with aminopolycarboxylates. J. Inorg. Nucl. Chem. 38, 617–621 (1976)
De Stefano, C., Milea, D., Pettignano, A., Sammartano, S.: Modeling ATP protonation and activity coefficients in NaClaq and KClaq by SIT and Pitzer Equations. Biophys. Chem. 121, 121–130 (2006)
Pitzer, K.S.: Activity Coefficients in Electrolyte Solutions, 2nd edn. CRC Press, Boca Raton (1991)
Bretti, C., Foti, C., Porcino, N., Sammartano, S.: SIT parameters for 1:1 electrolytes and correlation with Pitzer coefficients. J. Solution Chem. 35, 1401–1415 (2006)
Wright, M.R.: An Introduction to Aqueous Electrolyte Solutions. Wiley, London (2007)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Majlesi, K., Gholamhosseinzadeh, M. & Rezaienejad, S. Interaction of Molybdenum(VI) with Methyliminodiacetic Acid at Different Ionic Strengths by Using Parabolic, Extended Debye-Hückel and Specific Ion Interaction Models. J Solution Chem 39, 665–679 (2010). https://doi.org/10.1007/s10953-010-9531-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-010-9531-z