Abstract
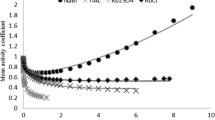
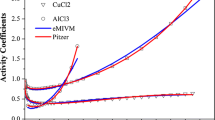
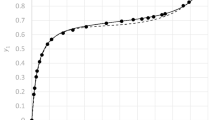
The empirical parameters of a two-parameter SIT equation were determined for some 1:1 electrolytes: chlorides, bromides, iodides, nitrates, perchlorates and carboxylates of alkali metals, inorganic acids and bases, and tetralkylammonium halides. A wide range of ionic strengths were considered. Canonical correlation analysis identified correlations between the SIT and Pitzer interaction coefficients for different classes of 1:1 type electrolytes.
Similar content being viewed by others
References
Bretti, C.; Foti, C.; Sammartano, S.: Calculation of SIT Parameters. Part I. A new approach in the use of SIT in determining the dependence on ionic strength of activity coefficients. Application to some chloride salts of interest in the speciation of natural fluids. Chem. Spec. Bioavail. 16, 105–110 (2004)
Biedermann, G.B.; Sillén, L.G.: Studies on the hydrolysis of metal ions. IV. Liquid junction potentials and constancy of activity factors. Ark. Kem. 5, 425–440 (1953)
Pitzer, K.S.: Thermodynamics of electrolytes. I. Theoretical basis and general equations. J. Phys. Chem. 77, 268–277 (1973)
Pitzer, K.S.: Activity Coefficients in Electrolyte Solutions, 2nd edn., CRC Press, Boca Raton, Florida (1991)
Brönsted, J.N.: Studies on solubility. IV. Principle of the specific interaction of ions. J. Am. Chem. Soc.44, 877–898 (1922)
Scatchard, G. : Concentrated solutions of strong electrolytes. Chem. Rev. 19 309–327 (1936)
Guggenheim, E.A.; Turgeon, J.C.: Specific interaction of ions. Trans. Faraday Soc. 51, 747–761 (1955)
Ciavatta, L.: The specific interaction theory in the evaluating ionic equilibria. Ann. Chim. (Rome) 70 551–562 (1980)
Ciavatta, L.: The specific interaction theory in equilibrium analysis. Some empirical rules for estimating interaction coefficients of metal ion complexes. Ann. Chim. (Rome) 80, 255–263 (1990)
Ögsthols, E.; Wanner, H.: The NEA Thermochemical Data Base Project. AEN-NEA, Issy-les-Moulineaux, France (2000)
Grenthe, I.; Wanner, V: Guidelines for the Extrapolation to Zero Ionic Strength. AEN-NEA, Issy-les-Moulineaux, France (2000)
Grenthe, I.; Puigdomenech, I.: Modelling in Aquatic Chemistry. OECD-NEA, Paris, France (1997)
Guggenheim, E.A.: Thermodynamics. Chapter 8, North Holland Publishing Co., Amsterdam (1957)
Millero, F.J.: Physical Chemistry of Natural Waters. Wiley Interscience, New York (2001)
Martell, A.E.; Smith, R.M.; Motekaitis, R.J.: National Institute of Standard and Technology, NIST. Critically Selected Stability Constants of Metal Complexes. PC-based Database, Gaithersburg, MD 20899 (2004)
Robinson, R.A.; Stokes, R.H.: Electrolyte Solutions. Butterworths Scientific Publications, London (1955)
Harned, S.H.; Owen, B.B.: The Physical Chemistry of Electrolytic Solutions. Reinhold Publishing Corporation, New York (1958)
Wen, W.Y.; Saito, S.; Lee, C.M.: Activity and osmotic coefficients of four symmetrical tetralkylammonium fluorides in aqueous solutions at 25°. J. Phys. Chem. 70, 1244–1248 (1966)
Bower, V.E.; Robinson, R.A.: Osmotic and activity coefficients of tetraethylammonium iodide in aqueous solution at 25°C. Trans. Faraday Soc. 59, 1717–1719 (1963)
Lindebaum, S.; Boyd, G.E.: Osmotic and activity coefficients for the symmetrical tetralkylaammonium halides in aqueous solution at 25°. J. Phys. Chem. 68, 911–917 (1964)
Bonner, O.D.: Osmotic and activity coefficients of the sodium salts of formic, acetic and propionic acids. J. Solution Chem. 17, 999–1002 (1998)
Rush, R.M.; Johnson, J.S.: Isopiestic measurements of the osmotic and activity coefficients for the systems HClO4-LiClO4-H2O, HClO4-NaClO4-H2O and LiClO4-NaClO4-H2O. J. Phys. Chem. 72, 767–774 (1968)
El Guendouzi, M.; Dinane, A.: Determination of water activities, osmotic and activity coefficients in aqueous solutions using the hygrometric method. J. Chem. Thermodyn. 32, 297–310 (2000)
El Guendouzi, M.; Marouani, M.: Water activities and osmotic and activity coefficients of aqueous solutions of nitrates at 25°C by the hygrometric method. J. Solution Chem. 32, 535–546 (2003)
El Guendouzi, M.; Dinane, A.; Mounir, A.: Water activity, osmotic and activity coefficients in aqueous chloride solutions at T = 298.15 K by the hygrometric method. J. Chem. Thermodynamics 33, 1059–1072 (2003)
Partanen, J.I.; Covington, A.K.: Re-evaluation of the activity coefficients of aqueous hydrochloric acid solutions up to a molality of 2.0 using two-parameter Hückel and Pitzer equations. Part I. Results at 25°C. J. Solution Chem. 31, 187–196 (2002)
Taghikhani, V.; Modarress, H.; Vera, J.H.: Individual anionic activity coefficients in aqueous electrolyte solutions of LiCl and LiBr. Fluid Phase Equil. 166, 67–77 (1999)
Torrent, J.; Sanz, F.; Virgili, J.: Activity coefficients of aqueous perchloric acid. J. Solution Chem. 15, 363–375 (1986)
Hamer, W.J.; Wu, Y.C.: Osmotic coefficients and mean activity coefficients of uni-univalent electrolytes in water at 25°C. J. Phys. Chem. Ref. Data 1, 1047–1099 (1972)
Anderson, T.W.: An Introduction to Multivariate Statistical Analysis. Wiley, New York (1958)
Forina, M.; Lanteri, S.; Armanino, C.: Q-Parvus, release 3.0: An extendable package of programs for esplorative data analysis, classification and regression analysis. Department of Chimica and Tecnologie Farmaceutiche, University of Genova. http://parvus.unige.it.
Grenthe, I.: Equilibrium Analysis, the Ionic Medium Method and Activity Factors, in Chemistry of Marine Waters and Sediments. In: Gianguzza, A.; Pellizzetti, E.; Sammartano S. (eds.) pp. 263–282, Springer-Verlag, Berlin (2002)
Grenthe, I.; Lagerman, B.: Ternary metal complexes. 2. The uranium(VI)-sulfate-hydroxide system. Radiochim. Acta 61, 169–176 (1993)
Author information
Authors and Affiliations
Corresponding author
Additional information
Calculation of SIT parameters: Part II. Part I, ref. [1].
Rights and permissions
About this article
Cite this article
Bretti, C., Foti, C., Porcino, N. et al. SIT Parameters for 1:1 Electrolytes and Correlation with Pitzer Coefficients. J Solution Chem 35, 1401–1415 (2006). https://doi.org/10.1007/s10953-006-9068-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-006-9068-3