Abstract
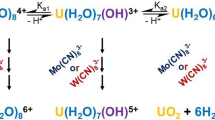
Hydroxamic acids are salt free, organic compounds with affinities for cations such as Fe3+, Np4+ and Pu4+, and have been identified as suitable reagents for the control of Pu and Np in advanced nuclear fuel reprocessing. The results of a UV-visible, near-IR spectrophotometric study of the 1:1 and 2:1 complexes formed between formo- and aceto-hydroxamic acids (FHA, AHA) and Np(IV) ions are interpreted using speciation diagrams for the identification of the species present at different pH and ligand to metal ratios. A kinetic model that describes the instability of the complex due to hydrolysis of the hydroxamate moiety, previously developed for the Fe(III)-AHA complexes (Andrieux et al. in J. Solution Chem. 36:1201–1217, [2007]), is tested here against experimental Np(IV)-FHA data. Consequently, the complexation constant for formation of the 1:1 Np(IV)-FHA complex in nitric acid is estimated at K 1=2715 and indications are that complexation protects the ligand against hydrolysis at 0.1>pH>−0.1.
Similar content being viewed by others
References
Andrieux, F.P.L., Boxall, C., Taylor, R.J.: The hydrolysis of hydroxamic acid complexants in the presence of non-oxidizing metal ions 1: Ferric ions. J. Solution Chem. 36, 1201–1217 (2007)
Dennis, I.S., Jeapes, A.P.: Reprocessing irradiated fuel. In: Wilson, P.D. (ed.) The Nuclear Fuel Cycle, Oxford Science Publications, Chap. 7, p. 116 (1996)
Birkett, J.E., Carrott, M.J., Fox, O.D., Jones, C.J., Maher, C.J., Roube, C.V., Taylor, R.J., Woodhead, D.A.: Recent developments in the Purex process for nuclear fuel reprocessing: Complexant based stripping for uranium-plutonium separation. Chimia 59, 898–904 (2005)
Taylor, R.J., May, I., Wallwork, A.L., Dennis, I.S., Hill, N.J., Galkin, B.Y., Zilberman, B.Y., Fedorov, Y.S.: The applications of formo- and aceto-hydroxamic acids in nuclear fuel reprocessing. J. Alloys Comp. 271–273, 534–537 (1998)
Birkett, J.E., Carrott, M.J., Fox, O.D., Jones, C.J., Maher, C.J., Roube, C.V., Taylor, R.J., Woodhead, D.A.: Controlling neptunium and plutonium within single cycle solvent extraction flowsheets for advanced fuel cycles. J. Nucl. Sci. Technol. 44, 337–343 (2007)
Taylor, R.J.: Progress towards understanding the interactions between hydroxamic acids and actinide ions. J. Nucl. Sci. Technol. Suppl. 3, 886–889 (2002)
Shackleford, S.: Development of an EQCM-based sensor for metal ions. PhD Thesis, University of Central Lancashire (2002)
Colston, B.J., Choppin, G.R., Taylor, R.J.: A preliminary study of the reduction of Np(VI) by formohydroxamic acid using stopped-flow near-infrared spectrophotometry. Radiochim. Acta 88, 329–334 (2000)
Taylor, R.J., Mason, C., Cooke, R., Boxall, C.: The reduction of Pu(IV) by formohydroxamic acid in nitric acid. J. Nucl. Sci. Technol. Suppl. 3, 278–281 (2002)
Chung, D.Y., Lee, E.H.: The reduction of Np(VI) by acetohydroxamic acid in nitric acid solution. In: Alvarez, R., Bryan, N.D., May, I. (eds.) Recent Advances In Actinide Science, pp. 587–589. Royal Society of Chemistry, Cambridge (2006)
Fox, O.D., Jones, C.J., Birkett, J.E., Carrott, M.J., Maher, C.J., Roube, C.V., Taylor, R.J.: Advanced PUREX flowsheets for future Np and Pu fuel cycle demands. In: Lumetta, G.J., Nash, K.L., Clark, S.B., Friese, J.I. (eds.) Separations for the Nuclear Fuel Cycle in the 21st Century. ACS Symposium Series, vol. 933, pp. 89–102. ACS, Washington (2006)
Todd, T.A., Wigelund, R.A.: Advanced separation technologies for processing spent nuclear fuel and the potential benefits to a geologic repository. In: Lumetta, G.J., Nash, K.L., Clark, S.B., Friese, J.I. (eds.) Separations for the Nuclear Fuel Cycle in the 21st Century. ACS Symposium Series, vol. 933, pp. 41–56. ACS, Washington (2006)
Taylor, R.J., May, I.: The reduction of actinide ions by hydroxamic acids. Czech J. Phys. 49, 617–621 (1999)
May, I., Taylor, R.J., Brown, G., Hill, N.J.: Np(IV) distribution between 30% tributyl phosphate in odourless kerosene and nitric acid. Radiochim. Acta 83, 135–138 (1998)
Taylor, R.J., Sinkov, S.I., Choppin, G.R., May, I.: Solvent extraction behaviour of neptunium ions in the presence of simple hydroxamic acids. Solv. Extr. Ion Exch. (2007, under review)
Danesi, P.R., Chiarizia, R., Scibona, G., D’Alessandro, G.: Stability constants of nitrate and chloride complexes of Np(IV), Np(V) and Np(VI) ions. J. Inorg. Nucl. Chem. 33, 3502–3510 (1971)
Phillips, R.J.: Chelating ion exchange with macroreticular hydroxamic acid resins. Ph.D. Thesis, Iowa State University, IS-T-910 (1980)
Monzyk, B., Crumbliss, A.L.: Acid dissociation constants (Ka) and their temperature dependencies (ΔHa, ΔSa) for a series of carbon- and nitrogen-substituted hydroxamic acids in aqueous solution. J. Org. Chem. 45, 4670–4675 (1980)
Friedman, H.A., Toth, L.M.: Absorption spectra of Np(III), (IV), (V) and (VI) in nitric acid solution. J. Inorg. Nucl. Chem. 42, 1347–1349 (1980)
Yoshida, Z., Johnson, S.G., Kimura, T., Krsul, J.R.: Neptunium. In: Morss, L.R., Edelstein, N.M., Fuger, J. (eds.) The Chemistry of the Actinide and Transactinide Elements, 3rd edn. Springer, Dordrecht (2006)
May, I., Taylor, R.J., Denniss, I.S., Brown, G., Wallwork, A.L., Hill, N.J., Rawson, J.M., Less, R.: Neptunium(IV) and uranium(VI) complexation by hydroxamic acids. J. Alloys Compd. 275–277, 769–772 (1998)
Ghosh, K.K.: Kinetic and mechanistic aspects of acid catalysed hydrolysis of hydroxamic acids. Indian J. Chem. 36B, 1089–1102 (1997)
Taylor, R.J., Boxall, C., Andrieux, F., Mason, C.: Modelling the hydrolysis of actinide complexed hydroxamic acid ligands. In: May, I., Alvarez, R., Bryan, N. (eds.) Recent Advances in Actinide Science, pp. 626–628. The Royal Society of Chemistry, Cambridge (2006)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Andrieux, F.P.L., Boxall, C., May, I. et al. The Hydrolysis of Hydroxamic Acid Complexants in the Presence of Non-Oxidizing Metal Ions 2: Neptunium (IV) Ions. J Solution Chem 37, 215–232 (2008). https://doi.org/10.1007/s10953-007-9225-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-007-9225-3