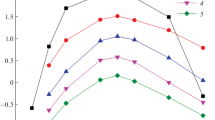
Abstract
Simple hydroxamic acids have been shown to have useful applications in an Advanced Purex process for the reprocessing of irradiated nuclear fuel. They are especially suited to the separation of neptunium (IV) from uranium (VI) by the selective formation of a hydrophilic complex with Np(IV). U(VI) extraction in to 30% tributyl phosphate is unaffected. However, they have also been shown to be very fast reducing agents for Np(VI). The timescales of the reduction have been defined under a range of typical Purex Process conditions although the accurate determination of the reaction kinetics was not possible due to the rapidity of the reaction. U(VI) was shown not to be reduced. Therefore, Np(VI) can be efficiently reductively stripped when solvent phase (30% tributyl phosphate in odourless kerosene) solutions of Np(VI) and U(VI) are contacted with aqueous phase hydroxamic acid solutions. The slow reduction of plutonium (IV) to Pu(III) has also been observed and this is apparently enhanced by the presence of U(VI) ions. The observed reactions of these actinide ions was shown to be compatible with experimentally determined onset potentials for hydroxamic acids. The hydrolysis of hydroxamic acids to hydroxylamine in nitric acid also affects the reduction of Pu(IV), particularly by FHA.
Similar content being viewed by others
References
I. S. Denniss and C. Phillips: in Solvent Extraction 1990, (Ed. T. Sekine), Elsevier, 1990.
P. Baron et al., in Proc. GLOBAL ’93, American Nuclear Society Inc., Illinois, 1993.
I. S. Denniss and A. P. Jeapes: in The Nuclear Fuel Cycle, (Ed. P. D. Wilson), Oxford Science Publications, Oxford, 1996.
R. J. Taylor, I. S. Denniss and A. L. Wallwork: Nuclear Energy 36 (1997) 39.
A. Riley, N. Donaldson and P. Parkes: in Proceedings RECOD ’98, forthcoming.
Richard J. Taylor, P. Wylie and P. Parkes: in Proc. Global ’97, 1997, 246.
R. J. Taylor et al.: in Proceedings RECOD ’98, forthcoming.
R. J. Taylor et al.: J. Alloys and Compounds, forthcoming.
I. May et al.: J. Alloys and Compounds, forthcoming.
I. May, R. J. Taylor and G. C. Brown: J. Alloys and Compounds, forthcoming.
R. J. Taylor, I. May, C. Boxall and C. Mason: Unpublished data.
J. D. Glennon, M. R. Woulfe, A. T. Senior and N. Nichoileain: Anal. Chem. 61 (1989) 1474.
J. V. Ramsbottom and N. A. Waters: J. Chem. Soc. B, 180 (1967).
D. W. M. Arrigan et al.: Analyst 118 (1993) 355.
A. J. Bard, R. Parsons and J. Jordan: Standard potentials in aqueous solution, Marcel Dekker, Inc., New York, 1985.
I. May, R. J. Taylor, I. S. Denniss and A. L. Wallwork: Czech. J. Phys., forthcoming.
G. S. Barney: J. Inorg. Nucl. Chem. 38 (1976) 1677.
R. J. Taylor et al.: Radiochim. Acta, forthcoming.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Taylor, R.J., May, I. The reduction of actinide ions by hydroxamic acids. Czech J Phys 49 (Suppl 1), 617–621 (1999). https://doi.org/10.1007/s10582-999-1041-0
Issue Date:
DOI: https://doi.org/10.1007/s10582-999-1041-0