Abstract
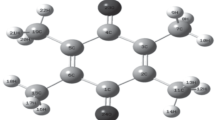
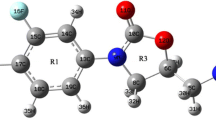
This work is devoted to theoretical study on molecular structure of protopine. The equilibrium geometry, harmonic vibrational frequencies and infrared intensities were calculated by ab initio Hartree-Fock and density functional B3LYP methods with the 6-31G(d) basis set and were interpreted in terms of potential energy distribution (PED) analysis. The internal coordinates were optimized repeatedly for many times to maximize the PED contributions. A detailed interpretation of the infrared spectra of protopine is reported. The calculations are in agreement with experiment. The thermodynamic functions of the title compound were also performed at HF/6-31G(d) and B3LYP/6-31G(d) level of theory. The FT-IR spectra of protopine were recorded in solid phase.
Similar content being viewed by others
References
B. Jiang, K. Cao, and R. Wang, Eur. J. Pharmacol., 506, 93–100 (2004).
D. Y. Wang, M. Z. Cheng, C. G. Wang, et al., Chin. J. Integr. Trad. Wes. Med., 6, 477–479 (1986).
V. N. Burtsev, E. N. Dormidontov, and V. N. Saliaev, Kardiologiia, 18, 76–79 (1978).
Z. A. Lu, D. C. Wan, Z. H. Chen, and X. H. Wang, Chin. Pharmaceut. J., 30, 81–84 (1992).
L. S. Song, G. J. Ren, Z. L. Chen, et al., Brit. J. Pharmacology, 129, 893–900 (2000).
Y. H. Huang, Z. Z. Zhang, and J. X. Jiang, Acta Pharmacol. Sinica, 12, 16–19 (1991).
R. X. Zhong, R. R. Shi, L. X. Huang, et al., Chin. Trad. Herb. Drugs., 17, 303–306 (1986).
C. X. Teng, Z. H. Chen, and G. S. Zhao, Acad. J. Kunming Med. Coll., 10, 44–46 (1989).
F. N. Ko, T. S. Wu, S. T. Lu, et al., Jpn. J. Pharmacol., 58, 1–9 (1992).
S. R. Kim, S. Y. Hwang, Y. P. Jang, et al., Planta Medica, 65, 218–221 (1999).
A. Z. Sadikov, B. Babaev, and T. T. Shakirov, Chem. Natur. Compounds., 10, No. 6, 852 (1976).
H. Takahashi, M. Iguchi, and M. Onda, Chem. Pharmaceut. Bull., 33, 4775–4782 (1985).
L. Dolejs, J. Slavik, and V. Hanus, Coll. Czechoslovak Chem. Commun., 29, No. 10, 2479 (1964).
J. Tousek, K. Malinakova, J. Dostal, and R. Marek, Magn. Reson. Chem., 43, No. 7, 578–581 (2005).
M. J. Frisch, G. W. Trucks, H. B. Schlegal, et al. Gaussian, Inc., Wallingford CT (2004).
H. B. Schlegel, J. Comput. Chem., 3, 214–218 (1982).
P. Hohenberg and W. Kohn, Phys. Rev., B136, 864–871 (1964).
A. D. Becke, J. Chem. Phys., 98, 5648–5652 (1993).
C. Lee, W. Yang, and R. G. Parr, Phys. Rev., B37, 785–789 (1988).
N. Sundaraganesan, H. Saleem, S. Mohan, and M. Ramalingam, Spectrochim. Acta, A61, 377–385 (2005).
N. Sundaraganesn, S. Ilakiamani, H. Saleem, et al., ibid., 2995–3001.
M. H. Jamroz Vibrational Energy Distribution Analysis: VEDA 4 Program, Warsaw (2004).
S. R. Hall and F. R. Ahmed, Acta Crystallogr., B24, 337–346 (1968).
P. L. Fast, J. Corchado, M. L. Sanches, and D. G. Truhlar, J. Phys. Chem., A103, 3139–3143 (1999).
V. Krishnakumar and R. J. Xavier, Spectrochim. Acta, A60, 709–714 (2004).
V. Krishnakumar and R. J. Xavier, Indian J. Pure Appl. Phys., 41, 597–601 (2003).
V. Krishnakumar, R. J. Xavier, and T. Chithambarathanu, Spectrochim. Acta, A62, 931–939 (2005).
S. J. Bunce, H. G. Edwards, A. F. Johnson, et al., ibid., A49, 775–783 (1993).
V. Krishnakumar and R. Ramasamy, ibid., A61, 673–683 (2005).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text Copyright © 2009 by S. A. Siddiqui, A. Dwivedi, P. K. Singh, T. Hasan, S. Jain, O. Prasad, and N. Misra
The text was submitted by the authors in English. Zhurnal Strukturnoi Khimii, Vol. 50, No. 3, pp. 433–442, May–June, 2009.
Rights and permissions
About this article
Cite this article
Siddiqui, S.A., Dwivedi, A., Singh, P.K. et al. Molecular structure, vibrational spectra and potential energy distribution of protopine using ab initio and density functional theory. J Struct Chem 50, 411–420 (2009). https://doi.org/10.1007/s10947-009-0062-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10947-009-0062-7