Abstract
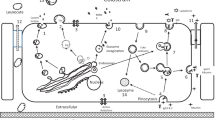
Biological transport of intact proteins across epithelial cells has been documented for many absorptive and secretory tissues. Immunoglobulins were some of the earliest studied proteins in this category. The transcellular transport (transcytosis) of immunoglobulins in neonatal health and development has been recognized; the process is especially significant with ungulates because they do not transcytose immunoglobulins across the placenta to the neonate. Rather, they depend upon mammary secretion of colostrum and intestinal absorption of immunoglobulins in order to provide intestinal and systemic defense until the young ungulate develops its own humoral defense mechanisms. The neonatal dairy calf’s ability to absorb immunoglobulins from colostrum is assisted by a ~24 h “open gut” phenomenon where large proteins pass the intestinal epithelial cells and enter the systemic system. However, a critical problem recognized for newborn dairy calves is that an optimum mass of colostrum Immunoglobulin G (IgG) needs to be absorbed within that 24 h window in order to provide maximal resistance to disease. Many calves do not achieve the optimum because of poor quality colostrum. While many studies have focused on calf absorption, the principal cause of the problem resides with the extreme variation (g to kg) in the mammary gland’s capacity to transfer blood IgG1 into colostrum. Colostrum is a unique mammary secretory product that is formed during late pregnancy when mammary cells are proliferating and differentiating in preparation for lactation. In addition to the transcytosis of immunoglobulins, the mammary gland also concentrates a number of circulating hormones into colostrum. Remarkably, the mechanisms in the formation of colostrum in ungulates have been rather modestly studied. The mechanisms and causes of this variation in mammary gland transcytosis of IgG1 are examined, evaluated, and in some cases, explained.










Similar content being viewed by others
Abbreviations
- IgG1:
-
Immunoglobulin G1
- IgA:
-
Immunoglobulin A
- IgM:
-
Immunoglobulin M
- FcRn:
-
Fc Receptor of the neonate
- Rab GTPases:
-
Rab25, Rab11a and Rab11b
- Rho-GTPases:
-
RhoB
- FcGRT:
-
Fc fragment of IgG Receptor Transporter alpha
- FcGRTsplice:
-
Splice variant of FcGRT missing trans-membrane domain
- β-2-M:
-
β-2-microglobulin
- MFGM:
-
Milk Fat Globule Membrane
- SAAM:
-
CONSAM software program
- E2:
-
17β-estradiol
- P4:
-
Progesterone
References
Neville MC, McFadden TB, Forsyth I. Hormonal regulation of mammary differentiation and milk secretion. J Mammary Gland Biol Neoplasia. 2002;7(1):49–66.
Brambell FW, Hemmings WA. The passage into the embryonic yolk-sac cavity of maternal plasma proteins in rabbits. J Physiol. 1949;108(2):177–85.
Brambell FW. The passive immunity of the young mammal. Biol Rev. 1958;33:488–531.
Brambell FW, Hennings WA, Morris IG. A theoretical model of gamma-globulin cataabolism. Nature. 1964;203:1352–4.
Simionescu M. In: Weissmann RdG, Samuelson B, Paoletti R, editors. Advances in inflammation research. Vol 1 ed. New York: Raven Press; 1979: 61–70.
Ollivier-Bousquet M. Milk lipid and protein traffic in mammary epithelial cells: joint and independent pathways. Reprod Nutr Dev. 2002;42(2):149–62.
Ollivier-Bousquet M. Transferrin and prolactin transcytosis in the lactating mammary epithelial cell. J Mammary Gland Biol Neoplasia. 1998;3(3):303–13.
Truchet S, Ollivier-Bousquet M. Mammary gland secretion: hormonal coordination of endocytosis and exocytosis. Animal. 2009;3(12):1733–42.
Wheeler TT, Hodgkinson AJ, Prosser CG, Davis SR. Immune components of colostrum and milk–a historical perspective. J Mammary Gland Biol Neoplasia. 2007;12(4):237–47.
Hanson LA. Comparative immunological studies of the immune globulins of human milk and of blood serum. Int Arch Allergy Appl Immunol. 1961;18:241–67.
Hanson LA. Comparative immune precipitin analysis of human milk and human blood plasma. Acta Pathol Microbiol Scand Suppl. 1961;51 Suppl 144:271–2.
Richards CB, Marrack JR. Sheep serum gamma globulin. In: Peeters H, editor. Protides of the biological fluids bruges. Amsterdam: Elsevier Science; 1963. p. 154–6.
Brandon MR, Watson DL, Lascelles AK. The mechanism of transfer of immunoglobulins into mammary secretion of cows. Aust J Exp Biol Med Sci. 1971;49(6):613–23.
Larson BL. Transfer of specific blood serum proteins to lacteal secretions near parturition. J Dairy Sci. 1958;41:1033–44.
Herr M, Bostedt H, Failing K. IgG and IgM levels in dairy cows during the periparturient period. Theriol. 2011;75:377–85.
Larson BL, Leary Jr HL, Devery JE. Immunoglobulin production and transport by the mammary gland. J Dairy Sci. 1979;63:665–71.
Sasaki M, Davis CL, Larson BL. Production and turnover of IgG1 and IgG2 immunoglobulins in the bovine around parturition. J Dairy Sci. 1976;59:2046–55.
Sasaki M, Larson BL, Nelson DR. Kinetic analysis of the binding of immunoglobulins IgG1 and IgG2 to bovine mammary cells. Biochim Biophys Acta. 1977;497:160–70.
Butler JE. Immunoglobulins of the mammary secretions. In: Larson BL, editor. Lactation: A comprehensive treatise. New York: Academic Press; 1974. p. 217–56.
Butler JE. Bovine immunoglobulins: an augmented review. Vet Immunol Immunopathol. 1983;41(2):43–152.
Hurley WL. Immunoglobulins of the mammary secretions. In: Fox PF, McSweeney PLH, editors. Advanced dairy chemistry: Proteins. 3rd ed. New York: Kluwer Academic/Plenum Publishers; 2003. p. 421–47.
Stelwagen K, Carpenter E, Haigh B, Hodgkinson A, Wheeler TT. Immune components of bovine colostrum and milk. J Anim Sci. 2009;87(13 Suppl):3–9.
Telemo E, Hanson LA. Antibodies in milk. J Mammary Gland Biol Neoplasia. 1996;1(3):243–9.
Zhang S, Mao Y, Huang J, Ma T, Zhang L, Zhu X, et al. Immunoglobulin gene locus events in epithelial cells of lactating mouse mammary glands. Cell Mol Life Sci. 2010;67:985–94.
Grosvenor CE, Picciano MF, Baumrucker CR. Hormones and growth factors in milk. Endocr Rev. 1993;14(6):710–28.
Nguyen DA, Neville MC. Tight junction regulation in the mammary gland. J Mammary Gland Biol Neoplasia. 1998;3(3):233–46.
Sordillo LM, Nickerson SC, Akers RM, Oliver SP. Secretion composition during bovine mammary involution and the relationship with mastitis. Int J Biochem. 1987;19:1165–72.
Sordillo LM, Doymaz MZ, Oliver SP. Morphological study of chronic Staphylococcus aureus mastitis in the lactating bovine mammary gland. Res Vet Sci. 1989;47(2):247–52.
Patton S, Keenan TW. The milk fat globule membrane. Biochim Biophys Acta. 1975;4115:273–309.
Zaczek M, Keenan TW. Morphological evidence for an endoplasmic reticulum origin of milk lipid globules obtained using lipid-selective staining procedures. Protoplasma. 1990;159:179–83.
Stemberger BH, Walsh RM, Patton S. Morphometric evaluation of lipid droplet associations with secretory vesicles, mitochondria and other components in the lactating cell. Cell Tissue Res. 1984;236:471–5.
Tzaban S, Massol RH, Yen E, et al. The recycling and transcytotic pathways for IgG transport by FcRn are distinct and display an inherent polarity. J Cell Biol. 2009;185(4):673–84.
Kacskovics I, Kis Z, Mayer B, et al. FcRn mediates elongated serum half-life of human IgG in cattle. Int Immunol. 2006;18(4):525–36.
Lu W, Zhao Z, Zhao Y, et al. Over-expression of the bovine FcRn in the mammary gland results in increased IgG levels in both milk and serum of transgenic mice. Immunology. 2007;122(3):401–8.
Andersen JT, Dalhus B, Cameron J, et al. Structure-based mutagenesis reveals the albumin-binding site of the neonatal Fc receptor. Nat Commun. 2012;3:610.
Nimmerjahn F, Ravetch JV. FcγRs in health and disease. In: Ahmed R, Honjo T, editors. Current topics in microbilogy and immunology. Berlin Heidelberg: Springer-Verlag; 2011. p. 105–25.
Barrington GM, McEwen BS, Huyler MT, Besser TE. Regulation of colostrogeneis in cattle. Livest Prod Sci. 2000;70:95–104.
Ghetie V, Ward ES. Multiple roles for the major histocompatibility complex class I- related receptor FcRn. Annu Rev Immunol. 2000;18:739–66.
Van Dyke RW. Acidification of lysosomes and endosomes. Subcell Biochem. 1996;27:331–80.
Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10(8):513–25.
Sönnichsen B, De Renzis S, Nielsen E, Rietdorf J, Zerial M. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J Cell Biol. 2000;149(4):901–14.
Espinosa EJ, Calero M, Sridevi K, Pfeffer SR. RhoBTB3: a Rho GTPase-family ATPase required for endosome to Golgi transport. Cell. 2009;137(5):938–48.
Fernandez-Borja M, Janssen L, Verwoerd D, Hordijk P, Neefjes J. RhoB regulates endosome transport by promoting actin assembly on endosomal membranes through Dia1. J Cell Sci. 2005;118(Pt 12):2661–70.
Stark A, Vachkova E, Wellnitz O, Bruckmaier R, Baumrucker C. Colostrogenesis: candidate genes for IgG(1) transcytosis mechanisms in primary bovine mammary epithelial cells. J Anim Physiol Anim Nutr (Berl). 2012 Dec 22. doi:10.1111/jpn.12021.
Baumrucker C, Bruckmaier R. Expression of bovine mammary FcGRT during lactation and involution. 2010. Unpublished Work.
Stark A, Wellnitz O, Dechow C, Baumrucker CR, Bruckmaier RM. Colostrogenesis during induced lactation in dairy cattle. Unpublished Data.
Ronge H, Blum JW. Somatomedin C and othegr hormones in dairy cows around parturition, in newborn calves and in milk. J Anim Physiol Anim Nutr. 1988;60:168–76.
Koldovsky O. Hormones in milk: their possible physiological significance for the neonate. In: Ledbenthal E, editor. Textbook of gastroenterology and nutrition in infancy. 2nd ed. New York: Raven Press; 1989. p. 97–119.
Peaker M, Neville MC. Hormones in milk: Chemical signals to the offspring. J Endocrinol. 1991;131:1–3.
Schams D, Karg H. Hormones in milk. Ann N Y Acad Sci. 1994;75–86.
Koldovsky O. Homromes in milk. Vitam Horm. 1995;50:77–149.
Campana WM, Baumrucker CR. Hormones and growth factors in bovine milk. In: Jensen RG, editor. Handbook of milk composition. San Diego: Academic Press; 1995. p. 476–94.
Blum JW, Baumrucker CR. Insulin-like growth factors (IGFs), IGF binding proteins, and other endocrine factors in milk: role in the newborn. Adv Exp Med Biol. 2008;606:397–422.
Baumrucker CR, Strbak V. Introduction to the symposium on hormones and bioactive substances in milk. Endocrin Reg. 1997;31:1–4.
Bartol FF, Wiley AA, Bagnell CA. Epigenetic programming of porcine endometrial function and the lactocrine hypothesis. Reprod Domest Anim. 2008;43 Suppl 2:273–9.
Grosvenor CE, Whitworth NS. Accumulation of prolactin by maternal milk and its transfer to circulation of neonatal rat - a review. Endocrinol Exp. 1983;17:271–82.
Blum JW, Baumrucker CR. Colostral and milk insulin-like growth factors and related substances: mammary gland and neonatal (intestinal and systemic) targets. Domes Anim Endocrinol. 2002;23:101–10.
Sylvia I Kehoe. Colostrum components and their impact on digestive function and growth of dairy calves The Pennsylvania State University, 2006. 1–130.
Baumrucker CR, Burkett AM, Magliaro-Macrina AL, Dechow CD. Colostrogenesis: mass transfer of immunoglobulin G1 into colostrum. J Dairy Sci. 2010;93(7):3031–8.
Kessler EC, Bruckmaier RM, Gross JJ. Milk production during the colostral period is not related to the later production level in dairy cows. Joint Annunal Meeting Abst# 493, 145. 2013.
Quigley III JD, Drewry JJ. Nutrient and immunity transfer from cow to calf pre- and postcalving. J Dairy Sci. 1998;81(10):2779–90.
Casey TM, Plaut K. The role of glucocorticoids in secretory activation and milk secretion, a historical perspective. J Mammary Gland Biol Neoplasia. 2007;12(4):293–304.
Quigley JD, Lago A, Chapman C, Erickson P, Polo J. Evaluation of the Brix refractometer to estimate immunoglobulin G concentration in bovine colostrum. J Dairy Sci. 2013;96(2):1148–55.
Rojas R, Apodaca G. Immunoglobulin transport across polarized epithelial cells. Nat Rev Mol Cell Biol. 2002;3(12):944–55.
Rodewald R. pH-dependent binding of immunoglobulins to intestinal cells of the neonatal rat. J Cell Biol. 1976;71(2):666–9.
Rodewald R, Lewis DM, Kraehenbuhl JP. Immunoglobulin G receptors of intestinal brush borders from neonatal rats. Ciba Found Symp. 1983;95:287–99.
Rodewald R, Kraehenbuhl JP. Receptor-mediated transport of IgG. J Cell Biol. 1984;99(1 Pt 2):159s–64s.
Baumrucker CR, Hammon H. Presence of FcRn in the intestine of newborn calves. 2013. Unpublished Work.
Lecce JG, Morgan DO. Effect of dietary regimen on cessation of intestinal absorption of large molecules (closure) in the neonatal pig and lamb. J Nutr. 1962;78:263–8.
Huber AH, Kelley RF, Gastinel LN, Bjorkman PJ. Crystallization and stoichiometry of binding of a complex between a rat intestinal Fc receptor and Fc. J Mol Biol. 1993;230(3):1077–83.
Kim J, Bronson CL, Wani MA, et al. Beta 2-microglobulin deficient mice catabolize IgG more rapidly than FcRn- alpha-chain deficient mice. Exp Biol Med (Maywood). 2008;233(5):603–9.
Ghetie V, Hubbard JG, Kim JK, Tsen MF, Lee Y, Ward ES. Abnormally short serum half-lives of IgG in beta 2-microglobulin-deficient mice. Eur J Immunol. 1996;26(3):690–6.
Kuo TT, Baker K, Yoshida M, et al. Neonatal Fc receptor: from immunity to therapeutics. J Clin Immunol. 2010;30(6):777–89.
Chaudhury C, Brooks CL, Carter DC, Robinson JM, Anderson CL. Albumin binding to FcRn: distinct from the FcRn-IgG interaction. Biochemistry. 2006;45(15):4983–90.
Chaudhury C, Mehnaz S, Robinson JM, et al. The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J Exp Med. 2003;197(3):315–22.
Schanbacher FL, Smith KL. Formation and role of unusual whey proteins and enzymes: relation to mammary function. J Dairy Sci. 1975;58(7):1048–62.
Monks J, Neville MC. Albumin transcytosis across the epithelium of the lactating mouse mammary gland. J Physiol. 2004;560(Pt 1):267–80.
Hunziker W, Kraehenbuhl JP. Epithelial transcytosis of immunoglobulins. J Mammary Gland Biol Neoplasia. 1998;3(3):287–302.
Giesecke WH, van den Heever LW. The diagnosis of bovine mastitis with particular reference to subclinical mastitis: a critical review of relevant literature. Onderstepoort J Vet Res. 1974;41(4):169–211.
Giesecke WH, Viljoen MH. The diagnosis of subclinical mastitis in lactating cows: a comparison of cytological methods and a monovalent radial immunodiffusion test. Onderstepoort J Vet Res. 1974;41(2):51–74.
Harman RJ, Schanbacher FL, Ferguson L, Smith KL. Changes in lactoferrin, immunoglobulin G, bovine serum albumin and a-lactoablumin during acute experimental and natural coliform mastitis in cows. Infect Immun. 1976;13:533–42.
Martin WL, West Jr AP, Gan L, Bjorkman PJ. Crystal structure at 2.8 A of an FcRn/heterodimeric Fc complex: mechanism of pH-dependent binding. Mol Cell. 2001;7(4):867–77.
Simister NE. Placental transport of immunoglobulin G. Vaccine. 2003;21(24):3365–9.
Leach JL, Sedmak DD, Osborne JM, Rahill B, Lairmore MD, Anderson CL. Isolation from human placenta of the IgG transporter, FcRn, and localization to the syncytiotrophoblast: implications for maternal-fetal antibody transport. J Immunol. 1996;157(8):3317–22.
Roopenian DC, Christianson GJ, Sproule TJ, et al. The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG-Fc-coupled drugs. J Immunol. 2003;170(7):3528–33.
Junghans RP, Anderson CL. The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proc Natl Acad Sci U S A. 1996;93(11):5512–6.
Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7(9):715–25.
Akilesh S, Christianson GJ, Roopenian DC, Shaw AS. Neonatal FcR expression in bone marrow-derived cells functions to protect serum IgG from catabolism. J Immunol. 2007;179(7):4580–8.
Cianga P, Medesan C, Richardson JA, Ghetie V, Ward ES. Identification and function of neonatal Fc receptor in mammary gland of lactating mice. Eur J Immunol. 1999;29(8):2515–23.
Cianga P, Cianga C, Cozma L, Ward ES, Carasevici E. The MHC class I related Fc receptor, FcRn, is expressed in the epithelial cells of the human mammary gland. Hum Immunol. 2003;64(12):1152–9.
Barrington GM, Besser TE, Davis WC, Gay CC, Reeves JJ, McFadden TB. Expression of immunoglobulin G1 receptors by bovine mammary epithelial cells and mammary leukocytes. J Dairy Sci. 1997;80(1):86–93.
Kemler R, Mossmann H, Strohmaier U, Kickhofen B, Hammer DK. In vitro studies on the selective binding of IgG from different species to tissue sections of the bovine mammary gland. Eur J Immunol. 1975;5(9):603–8.
Leary Jr HL, Larson BL, Nelson DR. Immunohistochemical localization of IgG1 and IgG2 in prepartum and lactating bovine mammary tissue. Vet Immunol Immunopathol. 1982;3(5):509–14.
Mayer B, Zolnai A, Frenyo LV, et al. Redistribution of the sheep neonatal Fc receptor in the mammary gland around the time of parturition in ewes and its localization in the small intestine of neonatal lambs. Immunology. 2002;107(3):288–96.
Mayer B, Doleschall M, Bender B et al. Expression of the neonatal Fc receptor (FcRn) in the bovine mammary gland. J Dairy Res 2005;72 Spec No:107–12.
Sayed-Ahmed A, Kassab M, bd-Elmaksoud A, Elnasharty M, El-Kirdasy A. Expression and immunohistochemical localization of the neonatal Fc receptor (FcRn) in the mammary glands of the Egyptian water buffalo. Acta Histochem. 2010;112(4):383–91.
Cervenak J, Kacskovics I. The neonatal Fc receptor plays a crucial role in the metabolism of IgG in livestock animals. Vet Immunol Immunopathol. 2009;128(1–3):171–7.
Doleschall M, Zhao Y, Mayer B, Hammarstrom L, Kacskovics I. Isolation of the gene encoding the bovine neonatal Fc receptor. Vet Immunol Immunopathol. 2005;108(1–2):145–50.
Yoshida M, Claypool SM, Wagner JS, et al. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity. 2004;20(6):769–83.
Yoshida M, Kobayashi K, Kuo TT, et al. Neonatal Fc receptor for IgG regulates mucosal immune responses to luminal bacteria. J Clin Invest. 2006;116(8):2142–51.
Baker K, Qiao SW, Kuo TT, et al. Neonatal Fc receptor for IgG (FcRn) regulates cross-presentation of IgG immune complexes by CD8-CD11b + dendritic cells. Proc Natl Acad Sci U S A. 2011;108(24):9927–32.
Kim J, Bronson CL, Hayton WL, et al. Albumin turnover: FcRn-mediated recycling saves as much albumin from degradation as the liver produces. Am J Physiol Gastrointest Liver Physiol. 2006;290(2):G352–60.
Kacskovics I, Wu Z, Simister NE, Frenyo LV, Hammarstrom L. Cloning and characterization of the bovine MHC class I-like Fc receptor. J Immunol. 2000;164(4):1889–97.
Werner-Misof C, Pfaffl MW, Meyer HHD, Bruckmaier RM. Effect of chronic oxytocin-treatment on the bovine mammary gland immune system. Vet Med. 2007;52:475–86. 102.
Einarsdottir HK, Selman MH, Kapur R, et al. Comparison of the Fc glycosylation of fetal and maternal immunoglobulin G. Glycoconj J. 2013;30(2):147–57.
Keenan TW, Morre DJ, Olson DE, Yunghans WN, Patton S. Biochemical and morphological comparison of plasma membrane and milk fat globule membrane from bovine mammary gland. J Cell Biol. 1970;44:80–93.
Keenan TW, Olson DE, Mollenhauer HH. Origin of the milk fat globule membrane. J Dairy Sci. 1970;54(3):295–9.
Winger K, Gay CC, Besser TE. Immunoglobulin G1 transfer into induced mammary secretions: the effect of dexamethasone. J Dairy Sci. 1995;78(6):1306–9.
Macrina AL, Tozer PR, Kensinger RS. Induced lactation in pubertal heifers: efficacy, response to bovine somatotropin, and profitability. J Dairy Sci. 2011;94(3):1355–64.
Kehoe SI, Jayarao BM, Heinrichs AJ. A survey of bovine colostrum composition and colostrum management practices on Pennsylvania dairy farms. J Dairy Sci. 2007;90(9):4108–16.
Berman M, Beltz WF, Greif PC, Chabay R, Boston RC. CONSAM User’s Guide. Washington, D.C. PHS Publ. # 1983-421-132:3279: U.S. Government Printing Office, 1983.
Green MH, Green JB. The application of compartmental analysis to research in nutrition. Ann Rev Nutr. 1990;10:41–61.
Friedman MH. Principles and models of biolgocial transport. 2nd ed. New York: Springer; 2008.
Gross J, Kessler EC, Bjerre-Harpoth V, Bruckmaier RM. Relevance of preipartal progesterone, prolactin profiles, and time of parturition on colostrum composition obtained at different stages of lactogenesis. J Dairy Sci. 2013; 96: E-Suppl 1: Abstract 493. Indianapolis, IN.
Zbinden RS, van Dorland HA, Remmelink C, Kemp B, van Knegsel TM, Bruckmaier RM. Effects of omitting the dry period on plasma progesterone and prolactin during lactogenesis and on colostrum IgG content in dairy cows. J Dairy Scie. 2013;96 E-Suppl.1, Abstract 324.
Annen EL, Fitzgerald AC, Gentry PC, et al. Effect of continuous milking and bovine somatotropin supplementation on mammary epithelial cell turnover. J Dairy Sci. 2007;90(1):165–83.
Guy MA, McFadden TB, Cockrell DC, Besser TE. Regulation of colostrum formation in beef and dairy cows. J Dairy Sci. 1994;77(10):3002–7.
Capuco AV, Ellis SE, Hale SA, et al. Lactation persistency: insights from mammary cell proliferation studies. J Anim Sci. 2003;81 Suppl 3:18–31.
Convey EM. Serum hormone concentrations in ruminants during mammary growth, lactogenesis, and lactation: a review. J Dairy Sci. 1974;57(8):905–17.
Tucker HA. Physiological control of mammary growth, lactogenesis, and lactation. J Dairy Sci. 1981;64(6):1403–21.
Willett LB, Smith KL, Schanbacher FL. Hormone induced lactation in the bovine III. Dynamics of injected and endogenous hormones. J Dairy Sci. 1976;59(3):504–14.
Smith KL, Schanbacher FL. Hormone induced lactation in the bovine. I. Lactational performance following injections of 17 beta-estradiol and progesterone. J Dairy Sci. 1973;56(6):738–43.
Lopez-Fontana CM, Maselli ME, Salicioni AM, Caron RW. The inhibitory effect of progesterone on lactogenesis during pregnancy is already evident by mid- to late gestation in rodents. Reprod Fertil Dev 2012. 24é704–14.
Caron RW, Jahn GA, Deis RP. Lactogenic actions of different growth hormone preparations in pregnant and lactating rats. J Endocrinol. 1994;142:535–45.
Lacasse P, Lollivier V, Dessauge F, Bruckmaier RM, Ollier S, Boutinaud M. New developments on the galactopoietic role of prolactin in dairy ruminants. Domest Anim Endocrinol. 2012;43(2):154–60. doi:10.1016/j.domaniend.2011.12.007. Epub 2012 Jan 10.
Brandon MR, Husband AJ, Lascelles AK. The effect of glucocorticoid on immunoglobulin secretion into colostrum in cows. Aust J Exp Biol Med Sci. 1975;53(1):43–8.
Barrington GM, Besser TE, Gay CC, et al. Regulation of the immunoglobulin G1 receptor: effect of prolactin on in vivo expression of the bovine mammary immunoglobulin G1 receptor. J Endocrinol. 1999;163(1):25–31.
Akers RM, Heald CW. Stimulatory effect of prepartum milk removal on secretory cell differentiation in the bovine mammary gland. J Ultrast Res. 1978;63:316–22.
Rijnkels M, Freeman-Zadrowski C, Hernandez J, et al. Epigenetic modifications unlock the milk protein gene loci during mouse mammmary gland development and differentiation. PLOS ONE. 2013;8:1–16.
Plaut K. Mammary gland blood flow. Personal communication. 2013
Koern M, Elston T, Blake W, Collins J. Stochasticity in gene expression: from theories to phenotypes. Nat Rev Genet. 2005;6:451–64.
Zhao X, Lacasse P. Mammary tissue damage during bovine mastitis: causes and control. J Anim Sci. 2008;86(13 Suppl):57–65. Epub 2007 Sep 4.
Watters RD, Guenther JN, Brickner AE, et al. Effects of dry period length on milk production and health of dairy cattle. J Dairy Sci. 2008;91(7):2595–603.
Annen EL, Collier RJ, McGuire MA, Vicini JL, Ballam JM, Lormore MJ. Effect of modified dry period lengths and bovine somatotropin on yield and composition of milk from dairy cows. J Dairy Sci. 2004;87(11):3746–61.
Gulay MS, Hayen MJ, Head HH, Wilcox CJ, Bachman KC. Milk production from Holstein half udders after concurrent thirty- and seventy-day dry periods. J Dairy Sci. 2005;88(11):3953–62.
Mansfeld R, Sauter-Louis C, Martin R. Effects of dry period length on milk production, health, fertility, and quality of colostrum in dairy cows. Invited review. Tierarztl Prax Ausg G Grosstiere Nutztiere. 2012;40(4):239–50.
Acknowledgments
The authors are grateful for the editorial suggestions and corrections provided by Dr. Ann Macrina and Nancy Baumrucker.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baumrucker, C.R., Bruckmaier, R.M. Colostrogenesis: IgG1 Transcytosis Mechanisms. J Mammary Gland Biol Neoplasia 19, 103–117 (2014). https://doi.org/10.1007/s10911-013-9313-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10911-013-9313-5